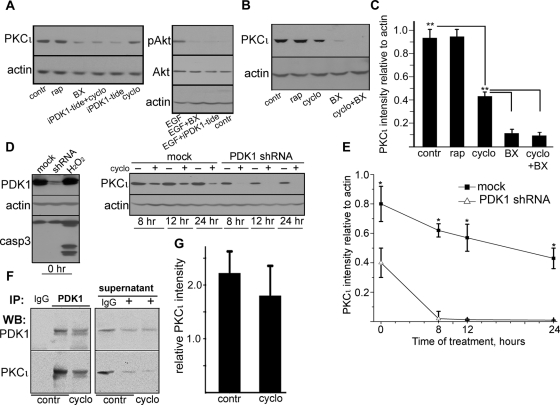
FIGURE 1:
PDK1 coimmunoprecipitates with and sustains steady-state levels of PKCι under protein synthesis inhibition. (A) Confluent, differentiated Caco-2 cells were treated with 10 μg/ml cycloheximide (cyclo), 100 nM rapamycin (rap; mTOR inhibitor), 0.5 μM BX-912 (BX; PDK1 inhibitor), 50 μM myristoylated PDK1 inhibitory pseudosubstrate peptide (iPDK1-tide), or none (contr) for 24 h. EGF-induced phosphorylation of Akt in parallel cultures was used as a positive control for the effect of both PDK1 inhibitors in the absence of cycloheximide. (B) In a similar experiment BX-912 was used in the presence of cycloheximide (last lane), or all three drugs were used separately. (C) The values from bands in three independent experiments as described in B were expressed as a ratio to the corresponding actin band (loading control) in the same lanes. Statistical significance was determined by Student's t tests of pairs of means; *p < 0.025 and **p < 0.005 indicate the probability of random differences from the average value immediately above (n = 3). (D) Caco-2 cells were transduced with mock lentiviral particles (mock) or with particles expressing anti-PDK1 shRNA and selected in puromycin. Confluent, differentiated cells not exposed to cycloheximide (0 h) were used to assess the efficacy of the knockdown and to control for apoptosis with anti–caspase 3 (casp3) antibody. A 2-h incubation in 20 mM H2O2 of mock cells served as a positive control for apoptosis. Cells were treated (+) or not (–) with 10 μg/ml cycloheximide for indicated periods of time for up to 24 h. Total SDS extracts were analyzed by immunoblotting with the antibodies indicated on the left. (E) The values from bands in three independent experiments as described in D were expressed as described in C and plotted as a function of time. (F) For coimmunoprecipitation experiments, Caco-2 cells were incubated or not (contr) with 10 μg/ml cycloheximide overnight (cyclo). The Triton-soluble fraction was immunoprecipitated with rabbit polyclonal anti-PDK1 antibody (+) or with nonimmune IgG, and analyzed by immunoblot for PDK1 or PKCι. The same blot analysis was performed for samples of the supernatant after the immunoprecipitation. (G) Relative amount of PKCι immunoprecipitated with PDK1 was calculated by normalizing the PKCι signal to the PDK1 signal in the same immunoprecipitates. Data represent the mean ± SD from three independent experiments. The averages of PKCι immunoprecipitated in the presence or absence of cycloheximide were not significantly different.