Abstract
World-wide population declines have sharpened concern for amphibian conservation on working landscapes. Across the Sierra Nevada's national forest lands, where almost half of native amphibian species are considered at risk, permitted livestock grazing is a notably controversial agricultural activity. Cattle (Bos taurus) grazing is thought to degrade the quality, and thus reduce occupancy, of meadow breeding habitat for amphibian species of concern such as the endemic Yosemite toad (Anaxyrus [ = Bufo] canorus). However, there is currently little quantitative information correlating cattle grazing intensity, meadow breeding habitat quality, and toad use of meadow habitat. We surveyed biotic and abiotic factors influencing cattle utilization and toad occupancy across 24 Sierra Nevada meadows to establish these correlations and inform conservation planning efforts. We utilized both traditional regression models and Bayesian structural equation modeling to investigate potential drivers of meadow habitat use by cattle and Yosemite toads. Cattle use was negatively related to meadow wetness, while toad occupancy was positively related. In mid and late season (mid July–mid September) grazing periods, cattle selected for higher forage quality diets associated with vegetation in relatively drier meadows, whereas toads were more prevalent in wetter meadows. Because cattle and toads largely occupied divergent zones along the moisture gradient, the potential for indirect or direct negative effects is likely minimized via a partitioning of the meadow habitat. During the early season, when habitat use overlap was highest, overall low grazing levels resulted in no detectable impacts on toad occupancy. Bayesian structural equation analyses supported the hypothesis that meadow hydrology influenced toad meadow occupancy, while cattle grazing intensity did not. These findings suggest cattle production and amphibian conservation can be compatible goals within this working landscape.
Introduction
Amphibian conservation is gaining considerable attention as a result of increasing quantitative evidence of global population declines [1], [2]. In the Sierra Nevada, nearly half of the native amphibian species are considered at risk by state and federal regulatory agencies [3], [4], . Exotic species introductions, infectious diseases, climate change, and anthropogenic land-use changes such as urbanization and agriculture have all been identified as potential drivers of amphibian declines [7]. Cattle grazing (Bos taurus), a prominent agricultural activity in the Sierra Nevada region, has received growing interest as a potential driver [8], [9], 10,11,12,13,14,15, and has been specifically implicated in amphibian species declines in the Sierra Nevada [4], [5], [6], [16].
One of the principal amphibian species of concern for the Sierra Nevada is the Yosemite toad (Anaxyrus [ = Bufo] canorus). Yosemite toad is an amphibian endemic to the Sierra Nevada, and is believed to have disappeared from approximately 50% of its known historic range [4], [17], [18]. Currently, Yosemite toad is a California Species of Special Concern, a U.S. Forest Service Sensitive Species (i.e., species that have exhibited downward trends in population numbers or in habitat capability, thus creating population viability concerns [19]), and a candidate species for federal listing pursuant to the Endangered Species Act [5], [6]. Yosemite toads are typically associated with upper montane and subalpine meadows (ca. 1,950 m to 3,450 m) in the central and southern Sierra Nevada [20], [21]. These mountain meadow habitats exhibit a range of hydrologic conditions varying from scattered, ephemeral pools to expansive, season-long flooded areas, which differentially support toad breeding and rearing habitats.
In addition to supplying vital wildlife habitat distinct from the surrounding forest matrix, mountain meadows also support a critical forage base for permitted cattle grazing in an otherwise depauperate zone [22], [23]. Permitted cattle grazing on the nation's public lands is a notably controversial activity, especially in high-elevation ecosystems. Public lands grazing permits often support low-intensity cattle operations on privately owned foothill ranches. Many Sierran ranching operations depend on these high-elevation rangelands during summer months, when low-elevation grasslands enter the inadequate dry forage period. During this inadequate period, low-elevation forage nutritive quality is generally poor and so managers must seek alternative feed sources (e.g., nutrient supplements, irrigated pastures, high-elevation pastures) to sustain livestock performance and the ranch enterprise [24]. Some suggest broad-scale reductions in public grazing permits would greatly impact the viability of these foothill ranches, forcing ranchers to sell land to developers, which has potentially negative regional socio-economic and ecological implications [25], [26]. However, opponents of public land grazing assert that cattle grazing has intolerable negative impacts on native wildlife and their habitat [27], [28].
In response to growing public concern surrounding cattle-amphibian interactions, some Sierra Nevada grazing permits have been terminated, and seasonal restrictions have been applied to many active permits with known populations of listed sensitive species. These types of management changes, directed to conserve species of concern, are often made despite considerable uncertainty about the system or with key quantitative information lacking. For example, within Sierra Nevada meadow systems, the extent to which cattle and amphibians, such as Yosemite toad, overlap in their habitat needs and use have not yet been jointly addressed. This is a critical knowledge gap because cattle grazing is more likely to have adverse effects if cattle tend to use similar sites as the species of concern, and less likely if they do not. Previous research on cattle-amphibian interactions is largely restricted to ungrazed and grazed (i.e., usually intensively or heavily grazed) comparisons [9], [13], [14], [29], which has limited relevance to systems experiencing extensive grazing (i.e., lower cattle intensities, largely unimproved native pasture systems), such as Sierra Nevada grazing allotments. Additionally, few analyses have applied a systems approach to examining these complex livestock-amphibian interactions at a management scale.
We surveyed meadow characteristics, cattle utilization, and Yosemite toad habitation across a set of Sierra Nevada meadows to simultaneously examine two potential drivers of meadow occupancy by toads: 1) cattle grazing intensity; and 2) meadow wetness (i.e., toad habitat quality). We utilized both traditional bivariate analyses and Bayesian structural equation modeling (SEM) [30], [31] to examine these proposed drivers of meadow occupancy by toads, in addition to potential meadow biotic and abiotic drivers of cattle utilization. SEM has become an effective tool for researchers working in inherently complex natural landscapes, providing greater systems level understanding than traditional approaches [32], [33], [34]. In this analysis, we explicitly asked: 1) how does meadow wetness influence forage quality and herbaceous biomass productivity? 2) what are the relationships between forage quality, forage productivity, and meadow utilization by cattle? and 3) what is the magnitude of influence of current cattle utilization versus meadow wetness on meadow occupancy by Yosemite toads? To address issues of the timing of grazing (i.e., with respect to the toad's lifecycle) that has greatest potential impact, these questions were examined within the seasonal periods in which grazing occurred: early, mid, and late season grazing periods (approximately mid-June through mid-July, mid-July through mid-August, and mid-August through mid-September, respectively).
Methods
Ethics statement
This observational field study was conducted in collaboration with the US Forest Service, and so all permissions for site access were granted and no permits were required. We employed sanitary protocols to reduce potential risks of spreading biological contaminants (e.g., the amphibian chytrid Batrachochytrium dendrobatidis) between meadows and watersheds. Prior to and following each meadow survey, crew members disinfected their rubber boots with a diluted bleach solution (4% sodium hypochlorite), which has been shown to cause 100% B. dendrobatidis mortality with as little as 30 seconds of exposure time [35].
Study area
This study was conducted on the High Sierra Ranger District of the Sierra National Forest, which is located on the western slope of the central Sierra Nevada in the upper montane zone (2 200 m to 2 700 m). The landscape is a mosaic of meadows, rock outcrops, and coniferous forest dominated by Pinus contorta, Pinus jeffreyi, Abies concolor, and Abies magnifica. Meadows, which cover less than 10% of the landscape, are generally characterized by shallow water tables (i.e., near-surface saturated conditions) and accumulations of mineral and organic materials. Within U.S. Forest Service (USFS) managed grazing allotments, 24 meadows providing potential toad breeding and rearing habitat were selected for study. Meadows spanned in elevation from 2 100 m to 2 700 m in elevation, and 0.3 ha to 7.9 ha in size. All meadows were open to cattle grazing under ambient USFS allotment scale management. Allotments ranged from 22 000 to 27 000 hectares with 200 to 250 permitted cow-calf pairs per allotment between mid-June and mid-September (Fig. 1). Soils were classified as Mollisols and Inceptisols with Histosols found in the most saturated zones of meadows [36]. Meadow vegetation was characterized by a dense cover of graminoid and herbaceous species. Meadows with near-surface saturated conditions throughout the growing season were generally dominated by sedges such as Carex utriculata, Carex vesicaria, and Carex simulata. In contrast, meadows experiencing seasonal water table drawdown below the rooting zone were generally dominated by grasses and forbs such as Deschampsia caespitosa and Trifolium species [36].
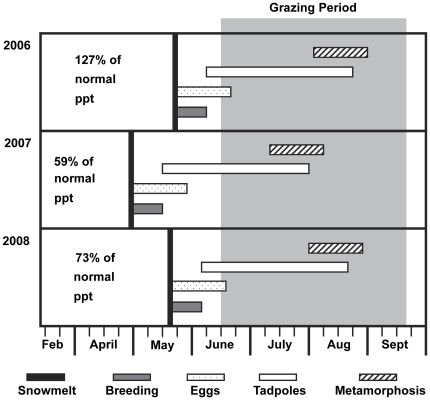
Figure 1. Life stage progression.
Diagram illustrating timing of Yosemite toad (Anaxyrus [ = Bufo] canorus Camp) life stages and cattle grazing seasons in the High Sierra Ranger District, Sierra National Forest, California, USA. Data were collected for 2006 to 2008 on cattle grazed meadows in the study area [77], [78].
Mean annual precipitation in the region is 115 cm, with 70% to 90% falling as snow from October through April. The growing season is relatively short— the region spends approximately 200 days under snowpack annually, with snowmelt typically occurring between May and June. Depending on snowpack depth and timing of melt, Yosemite toads typically emerge from hibernation in late spring (May to June) to breed and lay eggs in shallow pools and flooded areas of meadows. Larvae metamorphose by mid to late summer, and toad metamorphs remain within the breeding and rearing zone for the duration of the summer season [20], [21] (Fig. 1).
Study design and data collection
We conducted a cross-sectional, longitudinal survey of Yosemite toad occupancy, cattle utilization, vegetation attributes and meadow wetness across 24 meadows over three years (2006 to 2008) on the Sierra National Forest. For purposes of this study, toad occupancy was defined as evidence of breeding (i.e., presence of egg masses, tadpoles, and/or recent metamorphs). Yosemite toad and habitat survey records (conducted in 2002 and 2003; 83% and 94% of mean annual precipitation, respectively) from Forest staff were utilized to define an initial set of meadows with potential to support Yosemite toad breeding populations. From this initial set, we randomly sub-sampled 24 meadows across three grazing allotments. In 2006, five monitoring sites (120 total sites) were established in a stratified random approach across each meadow catena (i.e., a toposequence reflecting effects of topography on proximity to water table and on water movement), representing the major plant communities and moisture gradient in each meadow. Paired 1 m2 plots (one cattle grazed plot and one ungrazed caged plot) were randomly located within each plant community/moisture gradient monitoring site, with the ungrazed caged plots relocated within that same site in the second and third years [37], [38].
Cattle utilization and vegetation attributes were recorded at each monitoring site. Cattle utilization was measured via herbaceous utilization (2006 to 2008), which was determined by comparative yield-paired plot methods (Interagency 1996) at the end of the early (July), mid (August), and late (September) season grazing periods each year. In the final year of study (2008), fecal density was measured via three 35 m2 belt transects across each meadow to correlate annual utilization levels with a cumulative indicator of recent historic use (5 to 10 yrs). As a result of slow decomposition rates in high-elevation mountain systems, fecal density in mountain meadows represents approximately 5 to 10 years of pat accumulation.
Herbaceous biomass production data (2006 to 2008) and forage samples (2007 and 2008) were collected for each monitoring site in June, July, and August, representing variation in forage characteristics during early, mid, and late seasons, respectively. Herbaceous biomass production was determined via the comparative yield method at ungrazed caged plots [37]. For forage quality analyses, a minimum of 30 grams dry weight was sampled around each paired plot, representing the local plant community patch. Samples were oven-dried at 55°C to 60°C for a minimum of 48 hours, and ground to pass through a 40-mesh screen. Crude protein (CP), acid detergent fiber (ADF), and total phosphorous (TP) were determined for each sample by the University of California Agriculture and Natural Resources Analytical Laboratory, UC Davis, California. CP was directly calculated from sample nitrogen content, which was measured via nitrogen gas analyzer utilizing induction furnace and thermal conductivity [39]. ADF was determined gravimetrically as the residue remaining after acid detergent extraction [40]. For TP, samples were processed via nitric acid/hydrogen peroxide microwave digestion, and then TP was quantitatively determined by inductively coupled plasma atomic mission spectrometry [41], [42].
To assess overall meadow wetness, individual monitoring sites were categorized along a relative wetness scale with scores ranging from 0 to 6, as integers. In 2008, sites were ranked based on dominant plant community, extent and timing of surface flooding and saturation, and soil characteristics (mineral vs. organic dominated soils, depth of peat accumulation in organic soils, abundance of redox features in mineral soils). For example, relatively drier grass/forb-dominated sites on mineral soils represented a 0 rank, seasonally wet sites co-dominated by forb and Carex species common to moist sites (e.g., Aster alpigenus and Eleocharis species) represented a 3 rank, and continuously flooded sites dominated by wetland obligate Carex species represented a 6 rank. Site rankings were assigned at the end of the growing season (i.e., period of maximum water table draw down) so that rankings reflected relative differences between sites regardless of the type (wet, average, or dry) of rainfall year (i.e., ranks were on a fixed scale). Site rankings were averaged within each meadow to provide composite meadow-scale hydrologic rankings. For example, a meadow with a dominant wet Carex community and a subdominant drier grass/forb community would have three monitoring sites in the Carex community (3×6 rank) and two monitoring sites in the grass/forb community (2×0 rank), resulting in a mean score of 3.6, which is rounded to a “4” meadow rank assignment. Rankings were calibrated at sites within 10 additional meadows in the study allotments, which were equipped with ground water wells. Depth to free water was measured approximately every four weeks throughout the grazing season [43].
Meadow-scale toad occupancy surveys were conducted for all 24 meadows during the early tadpole periods (Fig. 1) in 2007 and 2008. Meadows were systematically searched for all toad life stages (egg masses, tadpoles, metamorphs, subadults, and adults) by three-member crews, with search times adjusted for individual meadow size and ease of search (e.g., more search time was allocated to meadows with high standing crop biomass). Searches were conducted during the early season (June–July), when tadpoles (i.e., the most easily detectable stage) were still abundant. Based on pilot studies, searches were conducted during mid-morning hours (0900–1100 hours) on cloudless days, which maximized detection potential. Each survey season, 5 of the 24 study meadows were completely resurveyed three times within a five day period to assess detection accuracy.
Data analysis
Bivariate relationships
In order to provide proof of concept, supporting the construction of the general conceptual diagram for structural equation analyses (see next section), we examined the following bivariate relationships via multiple regression analyses (i.e., generalized linear and linear models): 1) meadow wetness and toad meadow occupancy rates, peak biomass production, herbaceous biomass use, and fecal loading; 2) fecal pat density and herbaceous biomass use, and 3) forage quality metrics and meadow wetness. Meadow wetness was measured as the composite meadow-scale hydrologic rankings (see Study design and data collection section). We also used generalized linear models to examine potential bivariate relationships between toad occupancy rates and cattle utilization (i.e., total herbaceous utilization), and to investigate the possibility of an interaction between cattle utilization and meadow wetness in predicting toad occupancy rates. Site rankings used to calculate the composite meadow-scale hydrologic ranks were normally distributed. For the bivariate analyses, toad meadow occupancy rates were calculated as the proportion of surveys (three total, including preliminary Forest survey and 2007 and 2008 surveys) toads were observed in each meadow. For meadow wetness relationships, peak biomass production and late season herbaceous biomass use (i.e., total use) were averaged over 2006 to 2008 for each meadow. Fecal loading was calculated as fecal pat density in 2008, and was related to 2008 herbaceous biomass use. Forage quality metrics were averaged for 2007 and 2008 for each meadow and related to mean (2007 and 2008) late season herbaceous biomass use.
All regression analyses were conducted in STATA [44]. Because toad occupancy rate is a proportional response variable, fractional logistic regression models [45] were used to fit toad occupancy rates by meadow wetness and cattle utilization (i.e., total herbaceous utilization). For these generalized linear models, normality of deviance residuals [46] and Spearman rank correlation for the model predicted values and observed values [47] were utilized to assess general goodness of fit. The remaining bivariate relationships were fit with linear and quadratic regression models. AIC and significance tests were used to select final models. Standard diagnostic analyses were utilized to check assumptions of linearity, normality, and constant variance. Box-Cox transformations were used to remedy any violations [44].
Bayesian structural equation analyses
After conducting exploratory bivariate analyses, we used SEM to examine expected pathways between meadow wetness, cattle utilization, and toad occupancy of meadows. SEM is a multivariate analysis technique combining path and factor analyses that permits evaluation of potential causal pathways of intercorrelated variables [31], [48]. The Bayesian approach offers greater flexibility than classical frequentist approaches to SEM. Unlike classical maximum likelihood estimates, Bayesian inferences do not rely on asymptotic normality, and so these estimators are more reliable for smaller samples or cases with other sources of non-normality [30], [49].
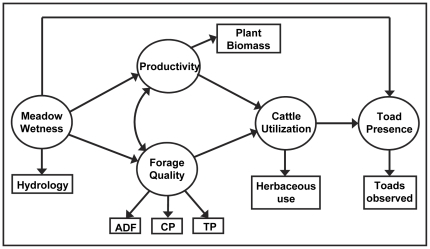
We began by constructing a conceptual SEM that incorporates the major known and hypothesized pathways of influence in the study system (Fig. 2). Within meadow ecosystems, it has been well established that spatio-temporal variation in depth to water tables exerts strong controls on plant community composition [22], [50]. Given this generally accepted relationship and the specific confirming results of above bivariate analyses, our conceptual SEM is based on the following: 1) via controls on community composition, meadow wetness influences plant community characteristics (i.e., productivity and forage quality), which are potentially correlated; 2) herbaceous biomass use by cattle is influenced by forage quality and productivity; 3) toad meadow occupancy is directly influenced by meadow wetness, which determines habitat suitability; and 4) toad meadow occupancy is directly influenced by cattle grazing (e.g., via impacts on physical and water quality attributes of toad habitat, trampling of individuals).
Figure 2. Conceptual Model.
Conceptual model of the multiple hypothesized factors influencing toad meadow occupancy in the High Sierra Ranger District, Sierra National Forest, California, USA. Ovals indicate latent variables, which are estimated by observable indicators, represented by boxes. Straight arrows represent direct effects of one variable on another and curved arrows represent correlations between variables.
For SEM analysis, we used logistic regression to model the binary (i.e., present/absent) response variable for toad occupancy and linear regression to model all other normally distributed variables within a hierarchical (i.e., multi-level) framework. To account for non-independence of repeated measurements within meadows, random effects (i.e., intercepts) for meadows were included in the models, and to account for possible higher-level grouping and elevation differences (enrolled grazing allotments spanned an elevation gradient), meadow effects were nested within grazing allotments. To account for possible mean differences among years, random effects for year were also included [51], [52], [53].
Bayesian SEM analysis was performed with OpenBUGS software, which uses Markov chain Monte Carlo (MCMC) simulation based on Gibbs sampling algorithm to fit the models [54]. For SEM, we analyzed herbaceous utilization, forage quality, biomass production, and meadow-scale toad occupancy data from 2007 and 2008 collection events, in addition to the one-time meadow hydrologic ranks. All continuous variables were standardized (mean = 0, standard deviation = 1) to aid model convergence and allow for direct comparisons of model coefficients. Model convergence was assessed utilizing trace plots with multiple chain sample values and a modified Gelman-Rubin statistic [55]. Model comparisons and goodness of fit were performed via the Deviance Information Criterion (DIC), a generalization of Akaike's Information Criterion (AIC) [56]. Statistical significance of individual model coefficients was examined via credible intervals (i.e., Bayesian equivalent of confidence intervals); coefficients were scored as significant when their 95% credible intervals excluded zero. To evaluate predictive capacity for toad occupancy and provide an additional measure of model fit, we cross-validated each model [57]. Each data point was held out and predicted by the model developed from the remaining n-1 data points via the R statistical package rjags [58], [59]. Prediction errors for toad occupancy were assessed via receiver operating characteristic (ROC) curves, which are widely used to assess performance of presence/absence models in habitat conservation research [60], [61]. The accuracy of the predictors is measured by the area under the ROC curve (AUC), which ranges from 0.5 (no better than random) to 1 (perfect). Although no standard classification rules exist, AUC values greater than 0.70 are generally considered good, and values greater than 0.90 are considered excellent [62].
Results
Conditions during study period
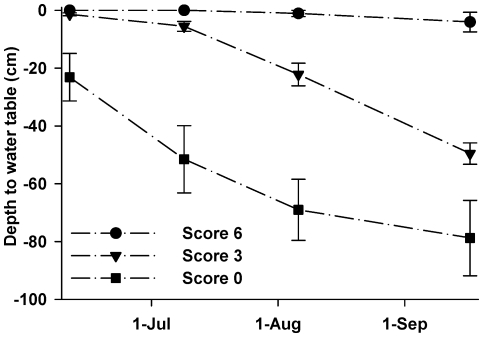
During the study period, annual precipitation was 146.5 cm in 2006 (127% of average), 68 cm in 2007 (59% of average), and 84.4 cm in 2008 (73% of average). For the overall study period (2006 to 2008), study meadows represented a mean annual cattle herbaceous vegetation use gradient from 4 to 49%, and an annual biomass production gradient from 1000 to 3200 kg•ha−1. Mean forage production for early, mid, and late seasons was 723 kg•ha−1 (+/−39 SE), 1660 kg•ha−1 (+/−127 SE), and 1774 kg•ha−1 (+/−98 SE), respectively. Meadow wetness scores sufficiently reflected the seasonal water table variation between meadow sites with “drier” (score 0), “moderately wet” (score 3), and “wettest” (score 6) hydrologic rankings in meadows equipped with ground water wells (Fig. 3). Water table depths diverged over a four month period (2008 year), with hydric sites remaining flooded throughout the season and drier sites experiencing a seasonal drawdown of approximately 55 cm. Repeated searches of meadows in both survey years resulted in zero false negatives, confirming that single mid-morning searches were sufficient in accurately detecting species presence. For each survey, meadows were designated as toad occupied if evidence of breeding was found (i.e., presence of egg masses, tadpoles, and/or recent metamorphs).
Figure 3. Water table dynamics.
Mean depth to water table by meadow hydrology score for 10 meadows in the High Sierra Ranger District, Sierra National Forest, California, USA, during 2008. Hydrologic scale ranged from 0 to 6, with 0 representing drier sites and 6 representing the wettest sites. Water tables diverged over the summer: wet sites (score 6) experienced a mean seasonal drawdown of 4 cm while drier sites (score 0) experienced an mean seasonal drawdown of 79 cm. Vertical bars represent ±1 SE.
Bivariate relationships
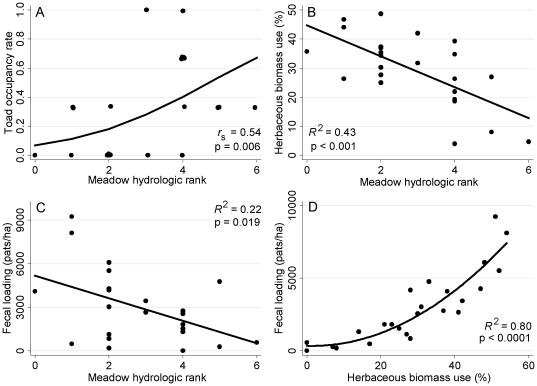
Toad meadow occupancy rates (out of 3 total surveys) were positively correlated with meadow wetness (fractional logistic model p = 0.006, Spearman rank correlation [rs] of predicted vs. observed values = 0.54; Fig. 4, panel A), while mean cattle utilization was negatively correlated with meadow wetness (herbaceous use: R 2 = 0.43, p = 0.0005; fecal pat density: R 2 = 0.22, p = 0.019; Fig. 4, panels B and C). Mean peak biomass production was also negatively correlated with meadow wetness (R 2 = 0.21, p = 0.026). In the fractional logistic regression model for toad occupancy rates, neither cattle utilization nor the interaction of cattle utilization by meadow wetness were significant (p>0.1). There was a strong, significant relationship (R 2 = 0.80, p<0.0001) between the 2008 late season use and fecal loading metrics (Fig. 4, panel D).
Figure 4. Toad and cattle meadow use.
Toad occupancy and annual cattle utilization (percent herbaceous biomass use and fecal pat density) along a hydrologic gradient of meadows (n = 24) in the High Sierra Ranger District, Sierra National Forest, California, USA, during 2006 to 2008. Toad occupancy rate is calculated as proportion of surveys (three total; 2002/2003, 2007, and 2008) each meadow was occupied.
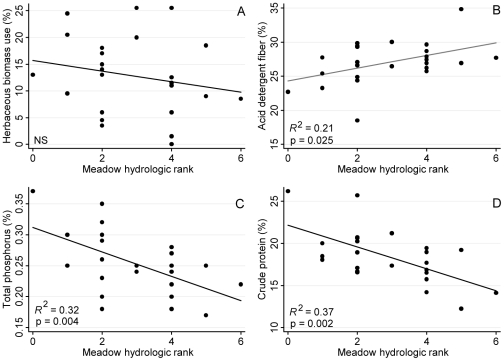
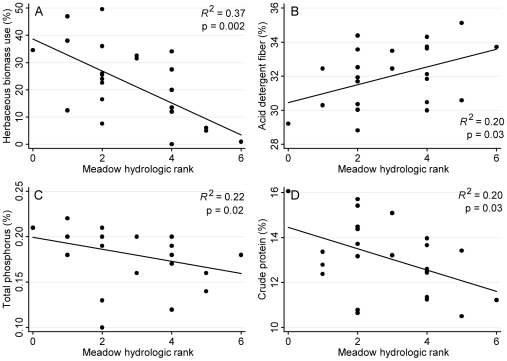
Analyses of the 2007 and 2008 cattle use and forage quality data revealed few differential relationships across the three grazing seasons. There was no significant relationship between herbaceous biomass use and meadow wetness during the early season (Fig. 5, panel A); however, there were significant negative relationships between meadow wetness and herbaceous biomass use for both mid and late seasons (Fig. 6, panel A; late season data not shown). For all grazing seasons, forage quality metrics (ADF, TP, CP) were negatively correlated with meadow wetness (Figs. 5 and 6; late season data not shown).
Figure 5. Early season bivariate analyses.
Early season (July) meadow scale cattle use and forage quality along a hydrologic gradient of meadows (n = 24) in the High Sierra Ranger District, Sierra National Forest, California, USA. There was no significant trend in cattle use, as measured by mean early season herbaceous biomass use, across the meadow hydrologic gradient (panel A). Forage quality (crude protein, total phosphorus [TP], acid detergent fiber [ADF]; panels B–D) significantly declined with increasing meadow hydrologic rank (i.e., meadow wetness).
Figure 6. Mid season bivariate analyses.
Mid season (August) meadow scale cattle use and forage quality along a hydrologic gradient of meadows (n = 24) in the High Sierra Ranger District, Sierra National Forest, California, USA. Cattle use, as measured by mean early season herbaceous biomass use (panel A), and mean forage quality (crude protein, total phosphorus [TP], acid detergent fiber [ADF; greater ADF values indicate lower digestibility]; panels B–D) significantly declined with increasing meadow hydrologic rank (i.e., meadow wetness). Late season (September) data exhibited similar trends.
Bayesian structural equation modeling
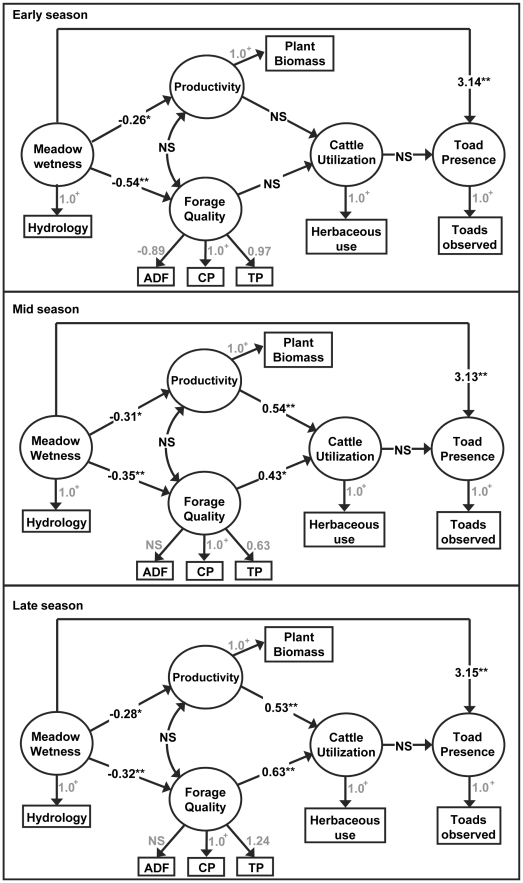
Bayesian SEM results for all grazing seasons suggest toad presence strongly responded to variation in meadow wetness, but did not respond to variation in cattle utilization (Fig. 7). Direct effects of cattle use on toad meadow occupancy were not significant (utilizing 90% Bayesian credible intervals) for any season. For all grazing seasons, meadow wetness significantly influenced forage quality and productivity, which were not significantly correlated (Fig. 7). Cross validations for toad occupancy predictions produced reasonably good ROC AUC values for all grazing seasons: early, mid, and late season model ROC AUC values were 0.830, 0.832, and 0.832, respectively. Along with the DIC indicators used to compare relative fit among models, these metrics indicate reasonable model fit.
Figure 7. Bayesian structural equation model analyses.
Results of Bayesian structural equation modeling for early, mid, and late season cattle use and forage data for the High Sierra Ranger District, Sierra National Forest, California, USA. All receiver operating characteristic (ROC) area under curve (AUC) values, which measured the accuracy of the predictors for toad occupancy, were ≥ 0.83. All models suggest toad presence responds to variation in meadow wetness, rather than cattle utilization levels. + = fixed values, ** = 95% Bayesian credible interval, * = 90% Bayesian credible interval, NS = Not significant.
Across the grazing seasons, cattle utilization responded differentially to meadow forage quality and productivity. Early season cattle utilization did not significantly respond to any of the measured forage quality or productivity indicators (i.e., plant biomass production, ADF, TP, or CP). During the early season, forage quality fully met the general nutrient requirements of CP and TP (approximately 8% and 0.20% respectively) for lactating beef cattle [63], and forage production was limited across meadows early in the herbaceous growing season. Productivity exhibited a greater relative effect (0.54 vs. 0.43) on cattle utilization during the mid grazing season, while forage quality had a greater relative effect (0.53 vs. 0.63) during the late grazing season. Comparing the relative importance of CP and TP as indicators of forage quality, CP was relatively more important (1.0 vs. 0.63) during the mid grazing season, while TP became relatively more important (1.24 vs. 1.0) during the late grazing season. Mean TP fell far below general nutrient requirements (mean = 0.136%, range = 0.076 to 0.174) during the late season. ADF was a significant indicator of forage quality only in the early season analysis. ADF values were generally low throughout the entire grazing season, ranging from 15% to 39%.
Discussion
Our study results suggest Yosemite toads and cattle largely select for divergent meadow types based on habitat and forage values, respectively (Figs. 4 and 7). Yosemite toads depend on meadows for vital breeding and rearing habitat, which is more abundant in wetter meadows. Wetter meadows provide greater habitat value for amphibians, which often exhibit metapopulation-like dynamics [64], [65], and potentially serve as source sites for overall population growth. Past habitat use surveys have shown that, in absence of cattle grazing, more than 50% of Yosemite toad subadults and adults are found in wet meadow bottoms, which provide persistent breeding and rearing pools [66]. These hydric zones are less likely to experience early season dry down (i.e., before tadpoles complete metamorphosis) than sites positioned higher in the meadow catena. Therefore, at the allotment scale, wetter meadows provide higher quality breeding and rearing habitat than relatively drier meadows, which provide more marginal habitat.
For cattle, wetter meadows provided relatively lower forage value for the majority of the grazing season. In the mid and late grazing seasons, cattle targeted relatively drier meadows, which supported more productive and nutritious plant communities, meeting general cattle nutrient requirements. As the grazing season progressed, forage quality became an increasingly important driver of cattle meadow selection as nutrient content declined with plant maturity (Fig. 7). However, during the early grazing season, forage quality was generally high and production was limiting across all meadows, resulting in relatively uniform grazing levels across all meadows. Despite these apparently uniform early season grazing levels (Fig. 5), cattle utilization did not significantly impact toad occupancy (Fig. 7). In this extensively grazed system, grazing intensities were light to moderate, with mean end of season use ranging from 4% to 49%. Fecal pat density, which serves as an indicator of longer term use patterns (i.e., given low environmental decomposition rates), was highly and significantly correlated with end of season cattle utilization (Fig. 4, panel D), indicating that use during the study period was indicative of cattle use over the past 5 to 10 years. Therefore, there are potentially two co-occurring mechanisms driving the overall lack of direct connection between cattle grazing and toad occupancy in this system: 1) for the majority of the grazing season, the two species mostly occupy differing zones along the moisture gradient, resulting in physical partitioning of the meadow habitat and minimizing any potential direct or indirect negative impacts; 2) when there is habitat use overlap (e.g., during the early part of the grazing season) grazing levels are low to moderate, resulting in no detectable impacts on toad occupancy.
Previous studies have reported negative associations between amphibian abundance and cattle grazing, indicating that amphibian species avoid or are excluded from livestock use areas. Following from this work, many have suggested cattle grazing activities reduce habitat value, citing potential mechanisms such as vegetation removal and degraded water quality [9], [11], [12], [13], [14], [29]. However, much of the existing cattle-amphibian work does not explicitly quantify grazing intensity, or has focused on grazed and ungrazed conditions in intensively grazed agro-ecosystems. Such comparisons generally offer limited application to extensive grazing systems, which commonly experience a continuum of grazing pressure. In a concurrent study within the same grazing allotments, our research group found no evidence that existing USFS grazing management impaired amphibian habitat conditions (i.e., water quality and cover) [67]. Other cattle-amphibian interaction studies from extensively grazed systems have demonstrated results similar to ours. In northeastern Oregon, an observational survey found no significant effects of extensive, moderate grazing on Columbia spotted frog (Rana luteiventris) reproduction [8]. Additionally, manipulative grazing experiments in the same region found no significant differences between grazed and ungrazed ponds in Columbia spotted frog egg mass counts, larval survival, or size at metamorphosis [68]. They also reported that nutrient levels were low or at minimum detection limits for all grazing and control treatments [68].
Our study clearly illustrates the importance of meadow wetness, and therefore hydrologic function, in determining toad occupancy. Loss of this critical wet meadow habitat will have direct negative impacts on Yosemite toad populations and other sensitive or threatened amphibian species. Some factors likely to negatively impact meadow hydrology and habitat availability include climate change, forest successional dynamics under altered natural fire regimes, and improper grazing management. Research at Yellowstone National Park has shown changes in climate (i.e., increased frequency and severity of drought, decreasing snowpack, and earlier runoff) and resultant wetland desiccation over the past 60 years were significantly correlated with declines in amphibian populations and species richness [69]. Climate models for the Sierra Nevada region suggest mountain meadows may be further threatened by predicted changes in future water yields, which will potentially result in overall longer periods of low flow conditions [70]. Shifts in both climate and fire regimes also alter forest successional dynamics, resulting in landscape-scale changes in vegetation cover [71], [72], which can potentially influence watershed-scale runoff and water yield [73]. Lastly, improper grazing management (e.g., heavy grazing, above levels reported in this study and above levels allowable by USFS regulations) can destabilize riparian areas and potentially lead to down-cutting and wetland desiccation via reduction in plant rooting mass and functional shifts in plant community composition [74], [75], [76]. Therefore, future habitat conservation practices for amphibian species of concern should focus on potentially critical factors directly impacting meadow hydrologic conditions, including climate change and land use activities such as heavy grazing, logging, and road construction.
Acknowledgments
We thank Neil McDougald and Dennis Dudley for sharing their expertise regarding range management, and for their help with data collection; Shannon Cler, Thomas Lushinsky, and Natalie Wegner for their help with data collection and sample processing; and Toby O'Geen, Valerie Eviner, and two anonymous reviewers for helpful comments in developing this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Research was funded by USDA Forest Service, Pacific Southwest Region. Other than providing preliminary data, the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Houlahan JE, Findlay CS, Schmidt BR, Meyer AH, Kuzmin SL. Quantitative evidence for global amphibian population declines. Nature. 2000;404:752–755. doi: 10.1038/35008052. [DOI] [PubMed] [Google Scholar]
- 2.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 3.CDFG 2009. Special animals (883 taxa). http://www.dfg.ca.gov/biogeodata/cnddb/plants_and_animals.asp.
- 4.Jennings MR, Hayes MP. Rancho Cordova: California Department of Fish and Game; 1994. Amphibian and reptile species of special concern in California: final report to California Department of Fish and Game, Inland Fisheries Division.255 [Google Scholar]
- 5.USDI USDI Fish and Wildlife Service. Endangered and threatened wildlife and plants; 12 month finding for petition to list Yosemite toad. Federal Register. 2002;67(237):75834–75843. [Google Scholar]
- 6.USDI Endangered and threatened wildlife and plants; review of species that are candidates or proposed for listing as endangered or threatened; annual notice of findings on resubmitted petitions; annual description of progress on listing actions. Federal Register. 2004;69(86):25876–24904. [Google Scholar]
- 7.Collins JP, Storfer A. Global amphibian declines: sorting the hypotheses. Divers Distrib. 2003;9:89–98. [Google Scholar]
- 8.Bull EL, Hayes MP. Livestock effects on reproduction of the Columbia spotted frog. J Range Manage. 2000;53:291–294. [Google Scholar]
- 9.Burton EC, Gray MJ, Schmutzer AC, Miller DL. Differential responses of postmetamorphic amphibians to cattle grazing in wetlands. J Wildlife Manage. 2009;73:269–277. [Google Scholar]
- 10.Denton JS, Hitchings SP, Beebee TJC, Gent A. A recovery program for the natterjack toad (Bufo calamita) in Britain. Conserv Biol. 1997;11:1329–1338. [Google Scholar]
- 11.Healey M, Thompson D, Robertson A. Amphibian communities associated with billabong habitats on the Murrumbidgee floodplain, Australia. Aust J Ecol. 1997;22:270–278. [Google Scholar]
- 12.Jansen A, Healey M. Frog communities and wetland condition: relationships with grazing by domestic livestock along an Australian floodplain river. Biol Conserv. 2003;109:207–219. [Google Scholar]
- 13.Jofre G, Reading C, di Tada I. Habitat selection in the Pampa de Achala toad, Bufo achalensis. Amphibia-Reptilia. 2007;28:129–138. [Google Scholar]
- 14.Knutson MG, Richardson WB, Reineke DM, Gray BR, Parmelee JR, et al. Agricultural ponds support amphibian populations. Ecol Appl. 2004;14:669–684. [Google Scholar]
- 15.Rannap R, Lohmus A, Jakobson K. Consequences of coastal meadow degradation: the case of the natterjack toad (Bufo calamita) in Estonia. Wetlands. 2007;27:390–398. [Google Scholar]
- 16.Jennings MR. Status of amphibians. In: Sierra Nevada Ecosystem Project: final report to Congress. Davis: University of California, Centers for Water and Wildland Resources. pp. 1996;921–944 [Google Scholar]
- 17.Drost CA, Fellers GM. Collapse of a regional frog fauna in the Yosemite area of the California Sierra Nevada, USA. Conserv Biol. 1996;10:414–425. [Google Scholar]
- 18.Kagarise Sherman C, Morton ML. Population declines of Yosemite toads in the eastern Sierra-Nevada of California. J Herpetol. 1993;27:186–198. [Google Scholar]
- 19.USDAForestService. 2005. 22 Forest Service Manual, FSM 2670.5. Washington, DC: USDA Forest Service National Headquarters (WO). Available at http://www.fs.fed.us/indirectives/fsm/2600/2670-2671.doc.
- 20.Karlstrom EL. The toad genus Bufo in the Sierra Nevada of California. UC Pubs in Zoology. 1962;62:1–104. [Google Scholar]
- 21.Kagarise Sherman C, Morton ML. The toad that stays on its toes. Nat Hist. 1984;93:72–78. [Google Scholar]
- 22.Allen-Diaz BH. Water table and plant species relationships in Sierra Nevada meadows. Am Mid Nat. 1991;126:30–43. [Google Scholar]
- 23.Ratliff R. Berkeley: USDA Forest Service; 1985. Meadows in the Sierra Nevada of California: state of knowledge. pp. PSW–GTR-84. 54 p. [Google Scholar]
- 24.George M, Bartolome JW, McDougald NK, Connor M, Vaughn C, et al. Oakland: Division of Agriculture and Natural Resources, University of California; 2001. Annual range forage production. [Google Scholar]
- 25.Huntsinger L, Forero L, Sulak A. Transhumance and pastoralist resilience in the western United States. 2010;1:1–15. [Google Scholar]
- 26.Sulak A, Huntsinger L. The importance of federal grazing allotments to central Sierran oak woodland permittees: a first approximation. In: Standiford RB, McCreary D, Purcell KL, editors. Proceedings of the fifth symposium on oak woodlands: oaks in California's challenging landscape General Technical Report PSW-184. Albany: USDA Forest Service, Pacific Southwest Research Station. pp. 2002;43–51 [Google Scholar]
- 27.Belsky AJ, Matzke A, Uselman S. Survey of livestock influences on stream and riparian ecosystems in the western United States. J Soil Water Conserv. 1999;54:419–431. [Google Scholar]
- 28.Fleischner TL. Ecological costs of livestock grazing in western North America. Conserv Biol. 1994;8:629–644. [Google Scholar]
- 29.Schmutzer AC, Gray MJ, Burton EC, Miller DL. Impacts of cattle on amphibian larvae and the aquatic environment. Freshwater Biol. 2008;53:2613–2625. [Google Scholar]
- 30.Congdon P. West Sussex: Wiley; 2003. Applied Bayseian Modeling.457 [Google Scholar]
- 31.Grace JB. Cambridge: Cambridge University Press; 2006. Structural Equation Moceling and Natural Systems. [Google Scholar]
- 32.Anderson TM, Ritchie ME, Mayemba E, Eby S, Grace JB, et al. Forage nutritive quality in the serengeti ecosystem: the roles of fire and herbivory. Am Nat. 2007;170:343–357. doi: 10.1086/520120. [DOI] [PubMed] [Google Scholar]
- 33.Grace JB, Pugesek BH. A structural equation model of plant species richness and its application to a coastal wetland. Am Nat. 1997;149:436–460. [Google Scholar]
- 34.Riginos C, Grace JB. Savanna tree density, herbivores, and the herbaceous community: bottom-up vs. top-down effects. Ecology. 2008;89:2228–2238. doi: 10.1890/07-1250.1. [DOI] [PubMed] [Google Scholar]
- 35.Johnson ML, Berger L, Philips L, Speare R. Fungicidal effects of chemical disinfectants, UV light, desiccation and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Organ. 2003;57:255–260. doi: 10.3354/dao057255. [DOI] [PubMed] [Google Scholar]
- 36.Roche LM. [Ph.D. Thesis], Davis, CA, University of California; 2011. Cattle grazing and provisioning of ecosystem services in Sierra Nevada mountain meadows. [Google Scholar]
- 37.Interagency. Utilization studies and residual measurements. In: BLM, editor. Interagency technical reference BLM/RS/ST-96/004+1730. Denver: USDI Bureau of Land Management's National Applied Resources Science Center. 1996:174. [Google Scholar]
- 38.Bonham CD. New York: John Wiley & Sons; 1989. Measurements for terrestrial vegetation. [Google Scholar]
- 39.AOAC AOAC official method 990.03, protein (crude) in animal feed, combustion method. Official methods of analysis of AOAC International. 18th ed. Gaithersburg: ASA-SSA Inc. pp. 2006;30–31 [Google Scholar]
- 40.AOAC AOAC official method 973.18, fiber (acid detergent) and lignin in animal feed. Official methods of analysis of AOAC International. 16th ed. Arlington: ASA-SSA Inc. pp. 1997;28–29 [Google Scholar]
- 41.Meyer GA, Keliher PN. An overview of analysis by inductively coupled plasma-atomic emission spectrometry. In: Montaser A, Golightly DW, editors. Inductively coupled plasmas in analytical atomic spectrometry. New York, NY: VCH Publishers. pp. 1992;473–516 [Google Scholar]
- 42.Sah RN, Miller RO. Spontaneous reaction for acid dissolution of biological tissues in closed vessels. Anal Chem. 1992;64:230–233. doi: 10.1021/ac00026a026. [DOI] [PubMed] [Google Scholar]
- 43.McIlroy SK. Identifying ecological patterns and processes in montane meadows of the Sierra Nevada Range [PhD]: University of California, Berkeley. 2008. 117
- 44.StataCorp . College Station: StataCorp LP; 2007. Stata statistical software: release 10. [Google Scholar]
- 45.Papke LE, Wooldridge JM. Econometric methods for fractional response variables with an application to 401(k) plan participation rates. J Appl Econom. 1996;11:619–632. [Google Scholar]
- 46.McCullagh P, Nelder JA. London: Chapman & Hall/CRC; 1989. Generalized linear models and extensions.511 [Google Scholar]
- 47.Aber JD, Magill AH. Chronic nitrogen additions at the Harvard Forest (USA): the first 15 years of a nitrogen saturation experiment. Forest Ecol Manag. 2004;196:1–5. [Google Scholar]
- 48.Bollen KA. New York: John Wiley and Sons; 1989. Structural equations with latent variables.514 [Google Scholar]
- 49.Lee S. West Sussex: Wiley; 2007. Structural equation modeling: a Bayesian approach.432 [Google Scholar]
- 50.Loheide SP, Deitchman RS, Cooper DJ, Wolf EC, Hammersmark CT, et al. A framework for understanding the hydroecology of impacted wet meadows in the Sierra Nevada and Cascade ranges, California, USA. 2009;17:229–246. [Google Scholar]
- 51.Gelman A, Hill J. Cambridge: Cambridge University Press; 2007. Data analysis using regression and multilevel/hierarchical models.607 [Google Scholar]
- 52.Pinheiro JC, Bates DM. New York: Springer-Verlag; 2000. Mixed-effects models in S and S-PLUS.528 [Google Scholar]
- 53.Rabe-Hesketh S, Skrondal A. College Station: Stata Press; 2008. Multilevel and longitudinal modeling using stata.562 [Google Scholar]
- 54.Thomas A, Hara BO, Ligges U, Sturtz S. R News; 2006. Making BUGS open. pp. 12–17. [Google Scholar]
- 55.Spiegelhalter D, Thomas A, Best N, Lunn D. 2007. OpenBUGS user manual, version 3.0.2. http://mathstat.helsinki.fi/openbugs/ManualsFrames.html.
- 56.Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. Bayesian measures of model complexity and fit. J Roy Stat Soc B. 2002;64:583–616. [Google Scholar]
- 57.Bagozzi RP, Yi Y. On the evaluation of structural equation models. J Acad Market Sci. 1988;16:74–94. [Google Scholar]
- 58.Plummer M. 2010. rjags: Bayesian graphical models using MCMC. Rpackage version 2.1.0-10. http://CRAN.R-project.org/package=rjags.
- 59.R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2010. R: A language and environment for statistical computing. [Google Scholar]
- 60.Carstensen B, Plummer M, Laara E, Hills M. 2010. Epi: A package for statistical analysis in epidemiology. R package version 1.1.17. http://CRAN.R-project.org/package=Epi.
- 61.Fielding AH, Bell JF. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv. 1997;24:38–49. [Google Scholar]
- 62.Ready J, Kaschner K, South AB, Eastwood PD, Rees T, et al. Predicting the distributions of marine organisms at the global scale. Ecol Model. 2010;221:467–478. [Google Scholar]
- 63.National Research Council. Washington, DC: National Academy Press; 1996. Nutrient requirements of beef cattle.248 [Google Scholar]
- 64.Alford RA, Richards SJ. Global amphibian declines: a problem in applied ecology. Annu Rev Ecol Syst. 1999;30:133–165. [Google Scholar]
- 65.Smith MA, Green DM. Dispersal and the metapopulation paradigm in amphibian ecology and conservation: are all amphibian populations metapopulations? Ecography. 2005;28:110–128. [Google Scholar]
- 66.Morton ML, Pereyra ME. Habitat use by Yosemite toads: life history traits and implications for conservation. Herpetol Cons Biol. 2010;5:388–394. [Google Scholar]
- 67.Roche LM, Allen-Diaz B, Eastburn DJ, Tate KW. Cattle grazing and Yosemite toad (Bufo canorus Camp) breeding habitat in Sierra Nevada meadows. Rangeland Ecol Manag. 2012;65:56–65. [Google Scholar]
- 68.Adams MJ, Pearl CA, McCreary B, Galvan SK, Wessell SJ, et al. Short-term effect of cattle exclosures on Columbia spotted frog (Rana luteiventris) populations and habitat in northeastern Oregon. J Herpetol. 2009;43:132–138. [Google Scholar]
- 69.McMenamin SK, Hadly EA, Wright CK. Climatic change and wetland desiccation cause amphibian decline in Yellowstone National Park. P Natl Acad Sci USA. 2008;105:16988–16993. doi: 10.1073/pnas.0809090105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Null SE, Viers JH, Mount JF. Hydrologic response and watershed sensitivity to climate warming in California's Sierra Nevada. PLoS ONE. 2010;5:1–16. doi: 10.1371/journal.pone.0009932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Norman SP, Taylor AH. Pine forest expansion along a forest-meadow ecotone in northeastern California, USA. Forest Ecol Manag. 2005;215:51–68. [Google Scholar]
- 72.Millar CI, Westfall RD, Delany DL, King JC, Graumlich LJ. Response of subalpine conifers in the Sierra Nevada, California, USA, to 20th-century warming and decadal climate variability. Arct Antarct Alp Res. 2004;36:181–200. [Google Scholar]
- 73.Bosch JM, Hewlett JD. A review of catchment experiments to determine the effect of vegetation changes on water yield and evapotranspiration. J Hydrol. 1982;55:3–23. [Google Scholar]
- 74.Kauffman JB, Krueger WC. Livestock impacts on riparian ecosystems and streamside management implications – a review. J Range Manage. 1984;37:430–438. [Google Scholar]
- 75.Sarr DA. Riparian livestock exclosure research in the western United States: a critique and some recommendations. Environ Manage. 2002;30:516–526. doi: 10.1007/s00267-002-2608-8. [DOI] [PubMed] [Google Scholar]
- 76.Toledo ZO, Kauffman JB. Root biomass in relation to channel morphology of headwater streams. J Am Water Resour As. 2001;37:1653–1663. [Google Scholar]
- 77.Lind AJ, Grasso R, Nelson J, Vincent K, Liang C. Vallejo: USDA Forest Service; 2011. Determining the effects of livestock grazing on Yosemite toads (Bufo canorus) and their habitat: final report addendum to USDA Forest Service Region 5.25 [Google Scholar]
- 78.Allen-Diaz B, Lind A, McIlroy S, Roche L, Grasso R, et al. Vallejo: USDA Forest Service; 2010. Determining the effects of livestock grazing on Yosemite Toads (Bufo canorus) and their habitat: final report to USDA Forest Service Region 5.45 [Google Scholar]