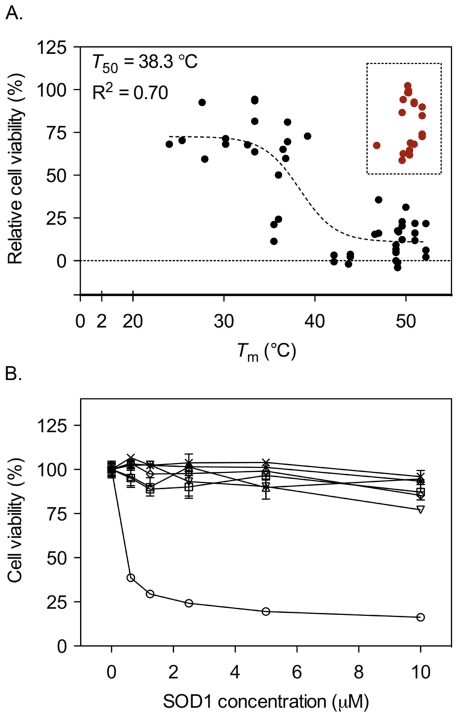
Figure 3. ApoSOD1 with perturbed Zn2+ site is non-toxic.
A set of apoSOD1 mutants with altered protein stability, charge and Zn2+ binding capacity was added to SH-SY5Y cells in duplicate or triplicate in a concentration range of 0.6 to 10 µM and incubated for 72 h. Cell viability was measured with the MTT assay. (A) Relative cell viability was calculated as described in methods, and plotted as a function of melting temperature, T m (Eq.5, Table 1). A correlation between cell viability and protein stability is observed; overall, the viability is higher for SOD1 mutants with low T m. SOD1 variants with mutated Zn2+ ligands as well as [F64A] and [D124G], all with a diminished ability to bind Zn2+, fall outside of this pattern (red symbols) (for detailed information, c.f. Table 1). The line represents a sigmoidal fit (Eq. 5) of all data points, except those from mutants with diminished ability to bind Zn2+. (B) Monomeric apoSOD1 (○) induces a toxic response, whereas [H63/71/80S;D83S] (□), [H71/80S;D83S] (▵), [H71/80S] (▿) and [H71S] (⋄) are all non-toxic. As all of these mutants lack H71, this amino acid was engineered back into [H63/71/80S;D83S] and [H71/80S;D83S], creating the mutants [H63/80S;D83S] (×) and [H80S;D83S] (✶). However, these mutants show no effect on cell viability, indicating that it is the concerted action of the Zn2+ ligands that underlies the toxicity, not H71 alone. Proteins were added in duplicate and data are presented as mean percentage of buffer control ± range.