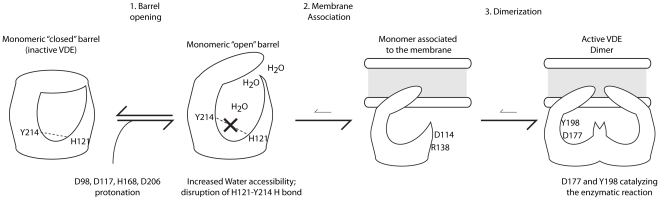
Figure 6. Model of VDE activation.
A model of VDE activation according to presented data is shown. The first step is the transition from the closed barrel structure (experimental inactive VDE) to a more open structure, where the cavity is more accessible to water and where the H121–Y214 hydrogen bond is destabilized. In a second step the open monomer associates to the membrane and successively dimerizes (thanks to interactions involving D114 and R138), leading to the final active conformation (active VDE). Here Y198 and D177 are available to catalyze the enzymatic reaction. In the absence of D98, D117, H168 and D206, the formation of active dimer is less efficient but possible since steps 2 and 3 are dependent from other residues.