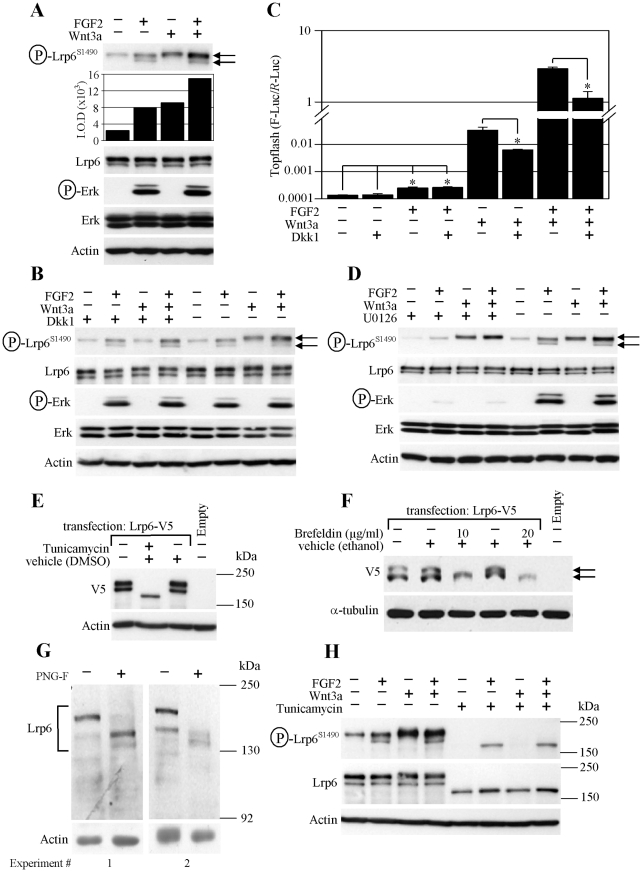
Figure 4. ERK phosphorylates LRP6 along its Golgi-based maturation pathway.
(A) The LRP6 phosphorylation induced in RCS cells by FGF2 and WNT3a was determined by WB and quantified by densitometry. LRP6 migrates as two bands, differently phosphorylated in cells treated by FGF2 and WNT3a (arrows). (B, C) Addition of recombinant DICKKOPF1 (Dkk1) prevents WNT3a-mediated LRP6 phosphorylation (B; upper LRP6 band) and Topflash activation (C), but not that induced by FGF2. (D) Inhibition of ERK pathway by U0126 (10 µM) prevents FGF2-mediated LRP6 phosphorylation (both bands; arrows) while it does not affect WNT3a-mediated phosphorylation (upper band). (E) RCS cells were transfected with V5-tagged LRP6 or empty plasmid, incubated in the presence of tunicamycin (0.1 µM) for 24 hours, and analyzed for indicated molecules by WB. (F) HEK293 cells were transfected with V5-tagged LRP6 plasmid and treated with brefeldin A for 24 hours. Empty vector (pcDNA3) serves as a control for transfection; ACTIN or α-TUBULIN serve as loading controls. LRP6 fails to fully mature in cells treated with tunicamycin or brefeldin A (arrows), which inhibit glycosylation or protein transport from the endoplasmatic reticulum to the Golgi network, respectively. (G) A whole RCS cell lysate was incubated with N-glycosidase F (PNG-F) for 16 hours and analyzed for LRP6 by WB. (H) RCS cells were cultivated with tunicamycin (5 µM) for 24 hours prior to FGF2 and WNT3a (1 hour) treatment. Note the lack of WNT3a-mediated LRP6 phosphorylation when only the immature, non-glycosylated LRP6 is produced as a result of tunicamycin treatment.