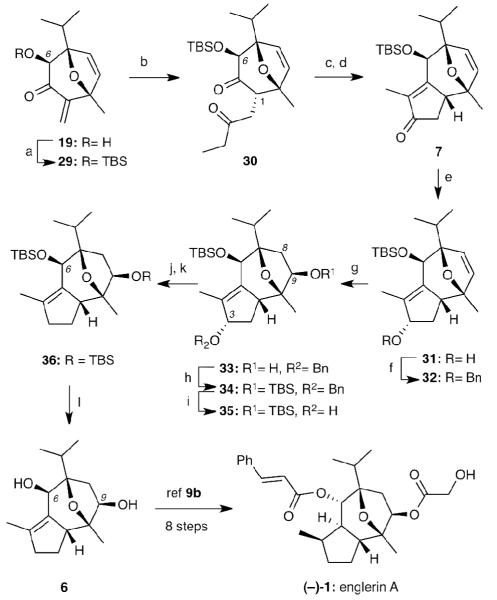
Scheme 7.
Reagents and conditions: (a) TBSOTf (2 equiv), NEt3 (5 equiv), CH2Cl2, 0°C to r.t. (b) propanal (4 equiv), 26 (20 mol%), Et3N, 80 °C, 75% over 2 steps; (c) NaHMDS (5 equiv), THF, 0 °C; (d) NaOMe (1.3 equiv), MeOH, 65 °C, 36%; 43% brsm for 2 steps; (e) NaBH4 (5 equiv), MeOH, rt; (f) NaH (4 equiv), BnBr (8 equiv), DMF, 60 °C, 71% for 2 steps; (g) BH3•THF (3 equiv), THF, rt then 3N NaOH/30% H2O2, 60%; (h) TBSOTf (2 equiv), Et3N (4 equiv), CH2Cl2, rt, 97%; (i) H2 (1 atm), Pd(OH)2 (catalytic), MeOH, rt, 99%; (j) Burgess reagent (5 equiv), PhMe, 80 °C, 90%; (k) H2 (1 atm), Pd/C (catalytic), MeOH, rt, 99%; (l) TBAF (3 equiv), THF, 80 °C, microwave, 45 min, 93%.