Scheme 9.
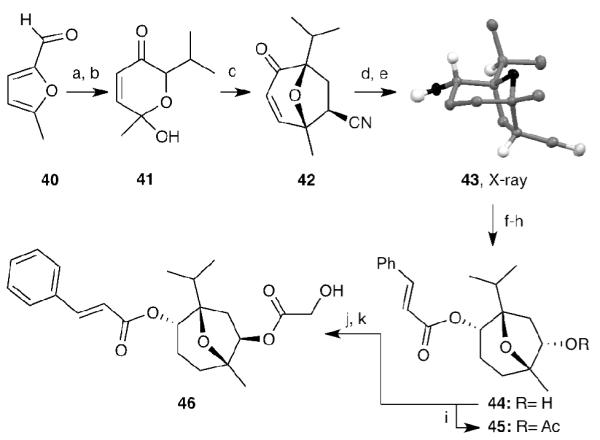
Reagents and conditions: (a) iPr2MgCl (1.5 equiv), Et2O, -10 °C; (b) mCPBA (1.0 equiv), CH2Cl2, 75% over two steps; (c) iPr2NEt (1.2 equiv), MsCl (1.2 equiv), acrylonitrile (80 equiv), 100 °C, microwave, 45%; (d) NaBH4 (1.0 equiv), CeCl3•H2O (1 equiv), MeOH, 0 °C, 98%; (e) H2, Pd/C (catalytic), EtOH, 99%; (f) LDA (2 equiv), THF, -78 °C, then O2, then SnCl2•HCl (30 equiv), 0 °C, 40%; (g) cinnamic acid (2.0 equiv), 2,4,6,-trichlorobenzoyl chloride (2.5 equiv), Et3N (3.0 equiv), 4-DMAP (catalytic), toluene, 95%; (h) NaBH4 (1.0 equiv), MeOH, 0 °C, 100%; (i) Ac2O (1.5 equiv), Et3N (4.0 equiv), 4-DMAP (0.5 equiv), CH2Cl2, r.t., 80%; (j) LiHMDS (1.3 equiv), Im2SO2 (1.3 equiv), THF, 0 °C to r.t., 99%; (k) Cesium hydroxy acetate (5 equiv), 18-crown-6 (5 equiv), toluene, 110 °C, 74%.
