Abstract
Objective
Enhanced adhesive signaling including activation of the focal adhesion kinase (FAK) is a hallmark of fibroblasts from lung fibrosis patients, and FAK has been therefore hypothesized to be a key mediator of this disease. This study was undertaken to characterize the contribution of FAK to the development of pulmonary fibrosis both in vivo and in vitro.
Methods
FAK expression and activity were analyzed in lung tissue samples from lung fibrosis patients by immunohistochemistry. Mice orally treated with the FAK inhibitor, PF-562,271, or with siRNA-mediated silencing of FAK, were exposed to intratracheally instilled bleomycin to induce lung fibrosis, and the lungs were harvested for histological and biochemical analysis. Using endothelin-1 (ET-1) as stimulus, cell adhesion and contraction, as well as profibrotic gene expression were studied in fibroblasts isolated from wild type and FAK-deficient mouse embryos. ET-1-mediated FAK activation and gene expression were studied in primary mouse lung fibroblasts, as well as in wild type and integrin β1-deficient fibroblasts.
Results
Increased FAK expression and activity are upregulated in fibroblast foci and remodeled vessels in lung fibrosis patients. Pharmacological or siRNA-mediated targeting of FAK resulted in marked abrogation of bleomycin-induced lung fibrosis. Loss of FAK impaired the acquisition of a profibrotic phenotype in response to ET-1. Profibrotic gene expression leading to myofibroblast differentiation required cell adhesion, and was driven by Jun N-terminal kinase activation through integrin β1/FAK signaling.
Conclusion
These results implicate FAK as a central mediator of fibrogenesis, and highlight this kinase as a potential therapeutic target in fibrotic diseases.
Fibrotic diseases, such as idiopathic pulmonary fibrosis (IPF) or scleroderma (SSc) are associated with high morbidity and mortality, and are unresponsive to currently available pharmacological therapies (1–3). Fibrosis is characterized by excess deposition and remodeling of the extracellular matrix (ECM) leading to organ failure and eventually to death. The fibrotic process is the result of an aberrant response to injury that induces the migration, proliferation, and activation of mesenchymal cells with the generation of myofibroblasts, the key players in tissue fibrogenesis (4). Thus, much interest exists, from both clinical and pharmaceutical points of view, in identifying mechanisms of inhibition of myofibroblast activity or function.
Myofibroblast differentiation is dependent on growth factors, matrix signaling and biomechanical tension (5, 6). It is now appreciated that activated mechanical loading and adhesive signaling is a key hallmark of fibrogenic responses, (7). Indeed, the basis of the myofibroblast phenotype is an increased ability to adhere to and contract ECM. These events are mediated by specialized cell surface structures termed focal adhesions, through which the contractile actin cytoskeleton is attached to the ECM (8). Integrins are the main cell surface receptors mediating cell–matrix communication in focal adhesions. We have reported that integrin β1, the integrin receptor mediating fibroblast attachment to fibronectin and collagens, is overexpressed in fibrotic fibroblasts, and that a neutralizing integrin β1 antibody reverses their excessive adhesion to and contraction of ECM (7). Recently, we also showed that genetic ablation of integrin β1 alleviates fibrosis in the bleomycin-induced skin fibrosis model (9, 10). Integrin β1 has been proposed to be involved in the development of pulmonary fibrosis by promotion of epithelial-to-mesenchymal transition (EMT) of alveolar epithelial cells (11). Additionally, it has been also reported that matrix stiffening regulates fibroblast activation by enhancing integrin-dependent mechanotransduction, with participation of integrin β1 (12). Nevertheless, further research is needed to enhance our understanding of the molecular mechanisms that control mechanical cues originating from the ECM or signals triggered by profibrotic mediators resulting in lung fibrogenesis.
Integrins mediate ECM-mediated adhesive signaling through the recruitment and activation of specific cytosolic proteins, for example, the focal adhesion kinase (FAK) (8). Aside from its well-established role in mediating integrin signaling, FAK may also participate in transduction pathways activated by growth factors via G protein–coupled receptors (GPCRs) and receptor tyrosine kinases (13–15). In this regard, FAK has been described to be an integrator of signals from profibrotic factors such as ET-1, connective tissue growth factor (CTGF) or transforming growth factor-β (TGF-β) (14–18). In fact, fibrotic cells often display persistently FAK activation and enhanced adhesion capacity (19, 20). In the present study, we have analyzed the contribution of FAK to the process of myofibroblast differentiation and fibrogenesis both in vitro and in vivo. Here, we present data showing that FAK expression and activity are upregulated in myofibroblast foci and highly-remodeled pulmonary arteries in lung tissue sections from lung fibrosis patients. We report also that pharmacological or genetic inactivation of FAK resulted in marked attenuation of bleomycin-induced lung fibrosis in a mouse model, without significantly affecting the initial inflammatory and vascular leakage responses. Using fibroblasts from wild type and FAK-deficient mouse embryos, we show that FAK is required for the acquisition of a profibrotic phenotype upon incubation with ET-1. Profibrotic gene expression leading to myofibroblast differentiation and fibrosis is driven by Jun N-terminal kinase (JNK) activation through integrin β1/FAK signaling. On the basis of these findings, we believe that FAK represents a crucial signaling molecule in myofibroblast differentiation and highlights FAK as a potential therapeutic target in pulmonary fibrosis.
Materials and Methods
An expanded version of Materials and Methods can be found as Supplemental Information.
Human lung tissue analysis
Lung tissue biopsy samples were obtained by open lung biopsy (thoracotomy) from 9 patients with pulmonary fibrosis (mean age, 52 ± 9 years; 3 females, 6 males), from which three presented connective tissue disease-associated pulmonary fibrosis (CTD-PF: 1 systemic sclerosis, 2 inflammatory myopathy with sclerodermatous changes), and the other 6 presented idiopathic pulmonary fibrosis (see Supplemental Table I). All of them were recruited and followed at the Rheumatology Department of the Hospital Universitario “12 de Octubre” (Madrid, Spain). Control samples were from 6 healthy subjects (organ donors not used for transplantation; mean age, 48 ± 14 years; 3 females, 3 males). Informed consent and ethical approval were obtained. Standard histological and immunohistochemical methodologies were used for the analysis of lung tissue.
Experimental model of bleomycin-induced lung fibrosis
Lung fibrosis was induced in C57BL/6 mice of 6–8 weeks of age (Jackson Laboratory) by application of bleomycin (Sigma) according to standard protocols. The extent of pulmonary fibrosis was assessed by standard histological and immunohistochemical methodologies. Bronchoalveolar lavage fluid (BALF) samples for analysis of leukocyte recruitment and vascular leakage were obtained as previously reported (21).
Cell culture
Mouse embryonic fibroblasts from wild type, as well as from FAK- and integrin β1-deficient mice were isolated and cultured by standard procedures (9, 16).
Immunoblotting analysis
For protein expression studies, fibroblasts were cultured in 6-well plates and processed essentially as previously described (22). Blots were probed with anti-α-SMA, anti-FAK, anti-phospho-FAK, anti-JNK, anti-phospho-JNK, anti-β-actin, anti-GAPDH, and anti- hemagglutinin [HA] antibodies.
Adhesion and floating collagen gel contraction assays
Fibroblast adhesion and floating collagen gel contraction experiments were performed as previously described (7, 22).
Analysis of mRNA expression by real time quantitative PCR and microarray experiments
RNA expression in mouse fibroblasts was analyzed by real time quantitative PCR (RT-qPCR) using TaqMan Assays on Demand (Applied Biosystems) according to protocols previously published (7). Microarray analysis were performed at the London Regional Genomics Centre (Robarts Research Institute, London, Ontario, Canada) using Affymetrix Mouse 430 2.0 arrays following manufacturer’s instructions.
Immunofluorescence microscopy
Detection of cellular vinculin and cytoskeletal actin filaments was performed by fluorescence microscopy according to protocols previously described (22).
Statistical analysis
Experimental data were analyzed by unpaired Student's t test in the case of normal distribution of data, or using nonparametric tests as appropriate. The P values obtained are indicated in the Figure legends when statistically significant.
Results
Increased FAK expression and activity is a hallmark of idiopathic pulmonary fibrosis
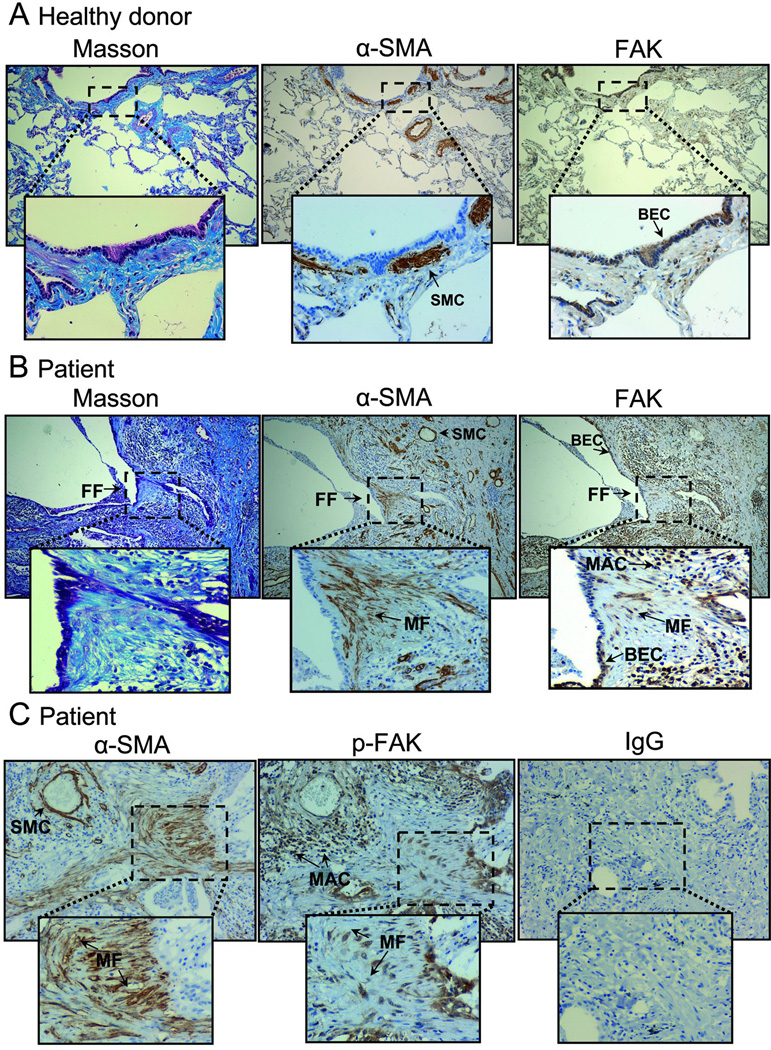
Several previous reports have pointed to the involvement of FAK in the development of fibrotic disorders (19, 23, 24). In order to extend these observations and analyze in depth the role of this kinase in human disease, we have studied the pattern of expression and activity of FAK in paraffin-embedded tissue sections from lung biopsies taken from pulmonary fibrosis patients (CTD-PF and IPF), and compared them with healthy donors. FAK is highly expressed in bronchial epithelial cells (BEC) in normal and fibrotic lungs (Fig.1A,B). Interestingly, FAK is also upregulated in a subset of myofibroblasts (MF) within fibrotic foci (FF) and infiltrating macrophages (MAC) present in the remodeled fibrotic lungs, as determined by immunohistochemical staining for α–SMA and FAK in serial histological sections (Fig.1B). Similarly, activated FAK (p-FAK: phospho-Y925) was detected in infiltrating macrophages and in myofibroblasts from fibrotic lungs (Fig.1C). No significant differences with respect to FAK or p-FAK staining were found in fibrotic tissue samples from CTD-PF and IPF. We have also carefully looked at the expression and activity of FAK in pulmonary vasculature. Lung fibrosis is usually associated to pulmonary hypertension and remodeling of pulmonary arteries (25). Here we show that tissue sections from pulmonary fibrosis patients displayed a marked hypertrophy of the smooth muscle in the media as detected with anti-α-SMA antibody, compared to controls (Suppl. Fig.1A,B). Interestingly, we detected strong FAK activity in the smooth muscle layer and also in endothelial cells in tissue sections from pulmonary fibrosis patients. A clear association between the thickening of the media layer and the extent of p-FAK was observed in IPF lungs (Suppl. Fig.1C). This staining pattern lends strong support to the notion that myofibroblasts within fibrotic foci and pulmonary vasculature highly upregulate FAK expression and activity and identifies FAK as a potential therapeutic target in human fibrotic diseases.
Figure 1. FAK expression and activity are upregulated in fibrotic foci in lungs from pulmonary fibrosis (PF) patients.
Lung tissue sections from control donors (A) and patients (B–C) were stained for Masson’s trichrome and for FAK, p-FAK and α-SMA. Sections from control donors showed the normal architecture of the lung tissue with restricted expression of α-SMA in bronchial and vascular smooth muscle cells (SMC) (A, left and middle). In contrast, Masson’s trichrome micrographs from patients revealed focal areas of collagen accumulation in the interstitium of major airways, blood vessels and alveolar septa (B, left). These fibrotic foci (FF) often contained an important number of α-SMA-positive myofibroblasts (MF) (B, middle). In healthy donors, overall FAK expression was low, except in bronchial epithelial cells (BEC) (A, right). A marked increase in FAK staining was observed in PF sections, localized mainly to BEC, but also to macrophages (MAC) (B, right), and α-SMA-positive MF within fibrotic foci (FF) (B, middle and left). p-FAK was detected in MF present in the fibrotic foci in PF tissue sections (C, middle). A control section without primary antibody is also shown (C, right). Microphotographs are representative of 8 different patients. Insets show selected regions at higher magnification.
Pharmacological and genetic inhibition of FAK ameliorates bleomycin-induced lung fibrosis
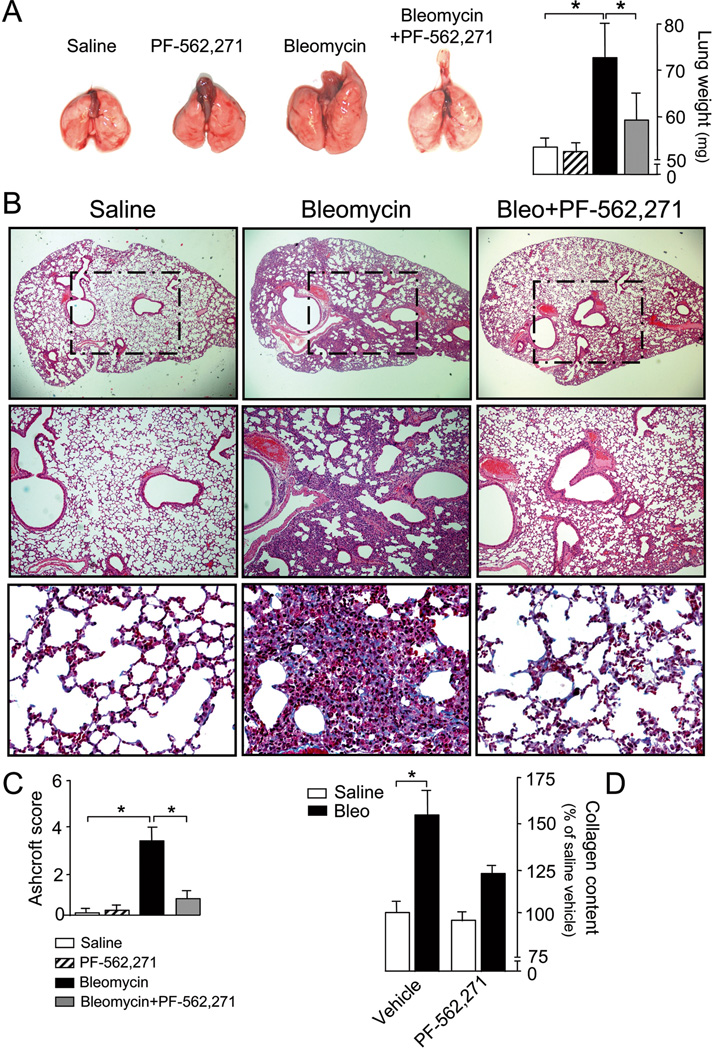
Pharmacological approaches that specifically target fibrogenic processes are currently lacking. In order to evaluate FAK as a potential therapeutic target for the treatment of lung fibrosis, we tested the effect of inhibiting FAK with the novel, ATP-competitive, reversible inhibitor of FAK (and Pyk2), PF-562,271 in the bleomycin-induced lung fibrosis model. At the employed dosage (15 mg/kg twice daily), PF-562,271 has been shown to effectively inhibit in vivo FAK activation (26–28). At day 21, lungs of mice receiving bleomycin were larger than those treated with saline, possibly as a consequence of the underlying tissue fibrosis (Fig.2A). Bleomycin-treated lungs also exhibited extensive areas of fibrosis with loss of the normal pulmonary architecture, intensively stained with Masson’s trichrome blue (Fig.2B). In contrast, PF-562,271-treated lungs showed a significantly better preservation of lung structures, similar to mice receiving saline. Semi-quantitative analysis of lung fibrosis using a modified Ashcroft scale and assessment of the collagen content confirmed these observations (Fig.2C,D).
Figure 2. In vivo FAK inhibition with PF-562,271 attenuates bleomycin-induced lung injury.
Mice receiving saline or bleomycin were systemically treated with PF-562,271 or vehicle. A) Representative photographs of whole lungs (left) and measurements of lung weight (right) on day 28 after treatment, n=8, *p <0.05. Black/white bars are explained in panel C. B) Lung fibrosis assessed by hematoxylin and eosin staining (upper and middle rows), and Masson’s trichrome blue staining (lower row). Micrographs are representative of at least eight independent experiments. C) Semi-quantitative analysis of lung injury using the Ashcroft score, n =8, *p <0.05. D) Acid-soluble collagen content in lung homogenates, n =8, *p <0.05.
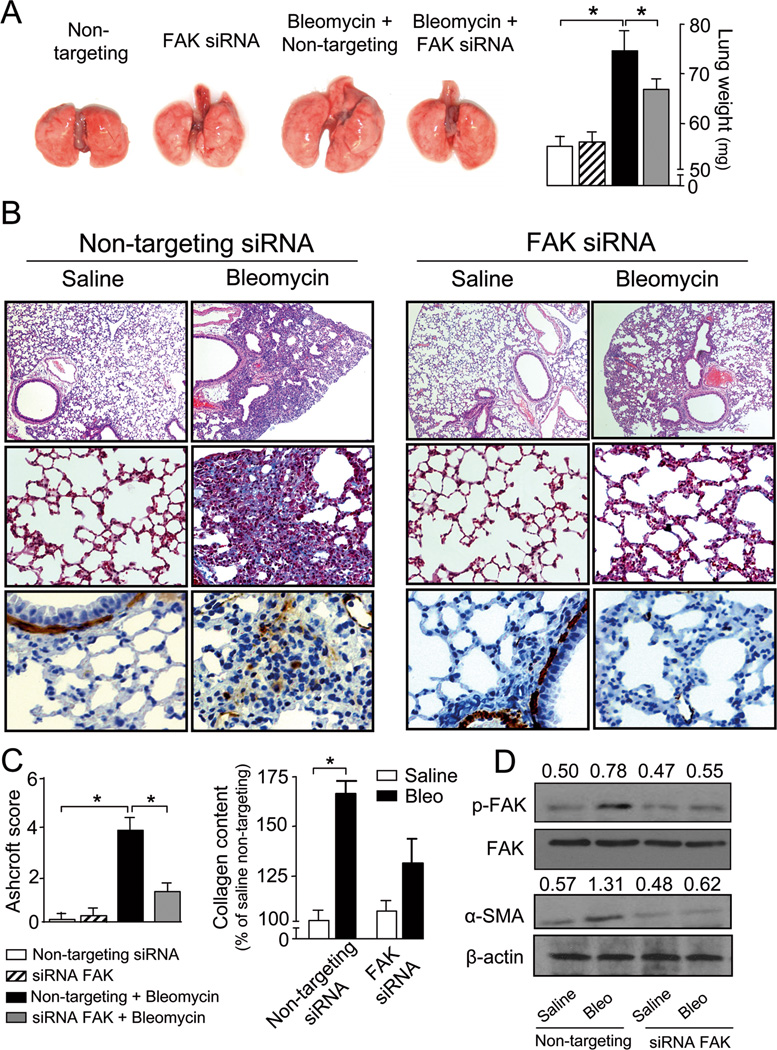
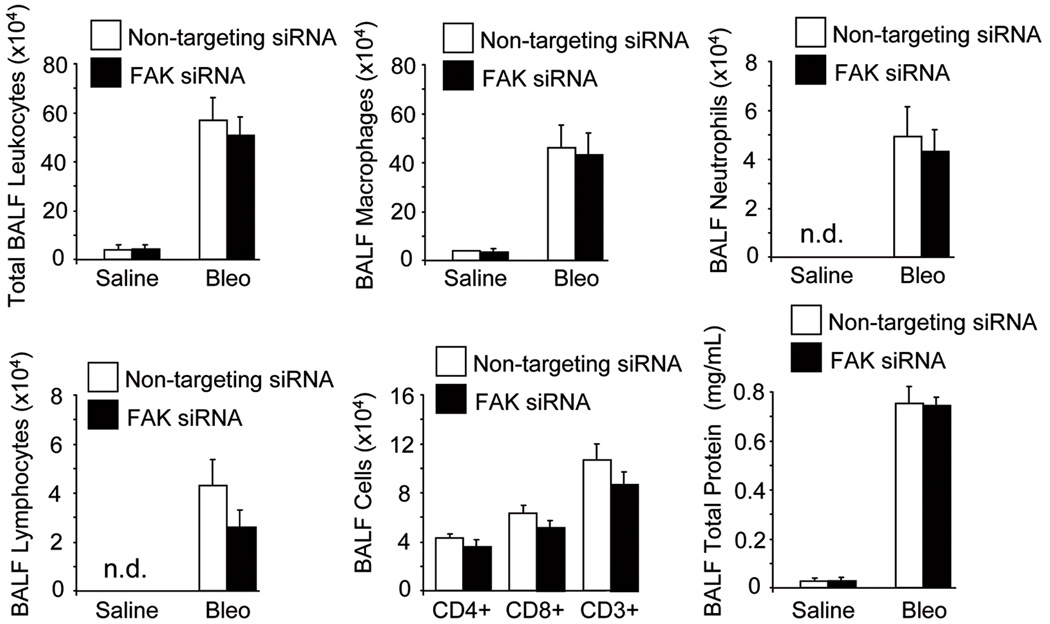
PF-562,271 is a dual FAK/Pyk2 inhibitor (28). Data from several reports indicate that the effects of FAK and Pyk2 can both converge, but may have differential origin and outcome (29). In order to confirm the specific involvement of FAK in the development of fibrosis, FAK expression was inhibited by the application of siRNA in the lungs of mice via intratracheal instillation. FAK siRNA effectively reduced FAK expression both in vitro and in vivo as assessed by immunoblotting. In vivo siRNA-mediated downregulation was reversible and FAK expression levels returned to control values 15 days after siRNA application (Suppl. Fig.2A,B). Compared to non-targeting, specific FAK siRNA treatment significantly abrogated lung fibrosis and markedly decreased ECM deposition, α-SMA-positive myofibroblast detection, and collagen content at 21 days post bleomycin. This was accompanied by a drastic reduction in the fibrotic score (Fig.3A–C). Interestingly, protein extracts of lungs obtained from bleomycin-treated mice under non-targeting siRNA displayed enhanced FAK phosphorylation and α-SMA expression compared to saline-treated mice, an effect that was absent in animals receiving FAK siRNA (Fig.3D), even when FAK expression levels recovered at 15 days of siRNA treatment (Suppl. Fig.2B). Lung injury triggers an initial tissue response characterized by inflammation and vascular leakage mediated by cytokines, chemokines and other inflammatory mediators released by damaged epithelial and/or endothelial cells (4). Thus, we sought to rule out the potential effect of FAK inhibition on the inflammatory response and vascular leakage induced by bleomycin lung injury. For that purpose, bronchoalveolar lavage fluids (BALF) of mice receiving saline or bleomycin at day 7 under non-targeting or specific FAK siRNA were obtained and tested for the presence and number of inflammatory cells and the release of total protein. As shown in Figure 4, bleomycin treatment induced an initial influx of inflammatory cells, an effect that often was correlated with enhanced fibrosis at later stages. Although the specific downregulation of FAK was protective from the fibrotic response, reduced FAK expression did not have any significant effect on the bleomycin-induced accumulation of inflammatory cells or total protein. Neither the number of total leukocytes nor that of macrophages, neutrophils or lymphocytes was altered by the silencing of FAK expression. Taken together, these results indicated that FAK inactivation abrogates lung fibrosis without significantly affecting the early inflammatory and vascular leakage response. We then focused our studies on the contribution of FAK to myofibroblast differentiation and behavior.
Figure 3. Down-regulation of FAK by siRNA prevents bleomycin-induced lung fibrosis.
FAK or control non-targeting siRNA were administered in a single dose to mice by intratracheal instillation together with bleomycin or saline. A) Representative photographs of whole lungs (left) and measurements of lung weight (right) on day 21 after treatment, n =8, *p <0.05. Black/white bars are explained in panel C. B) Lung fibrosis assessed by hematoxylin and eosin staining (upper row), Masson’s trichrome blue staining (middle row) and immunohistochemical detection of α-SMA-positive myofibroblasts (lower row). Micrographs are representative of at least eight independent experiments. C) Semi-quantitative analysis of lung injury using the Ashcroft score, n =8, *p <0.05 (left) and acid-soluble collagen content in lung homogenates, n =8, *p <0.05 (right). D) Detection of p-FAK, FAK, α-SMA and β-actin in lung lysates of mice receiving bleomycin or saline under non-targeting or FAK siRNA treatment. Values above the blots refer to the ratio of the signal p-FAK/FAK or α-SMA/β-actin after densitometric analysis, n=3.
Figure 4. Vascular leak and leukocyte recruitment and activation induced by bleomycin injury is unaltered in siRNA FAK-treated mice.
BALF samples were obtained from bleomycin and saline-treated mice and analyzed by cytospin and cytofluorometry, as well as for total protein content. BALF total numbers of leukocytes, macrophages, neutrophils, lymphocytes, and CD4+, CD8+ and CD3+ T cells (only bleomycin-treated mice), n=4. Assessment of lung vascular permeability by BALF total protein determination, n=4. Data presented are from one of two independent experiments with similar results (n.d. not detected).
Loss of FAK impairs myofibroblast differentiation
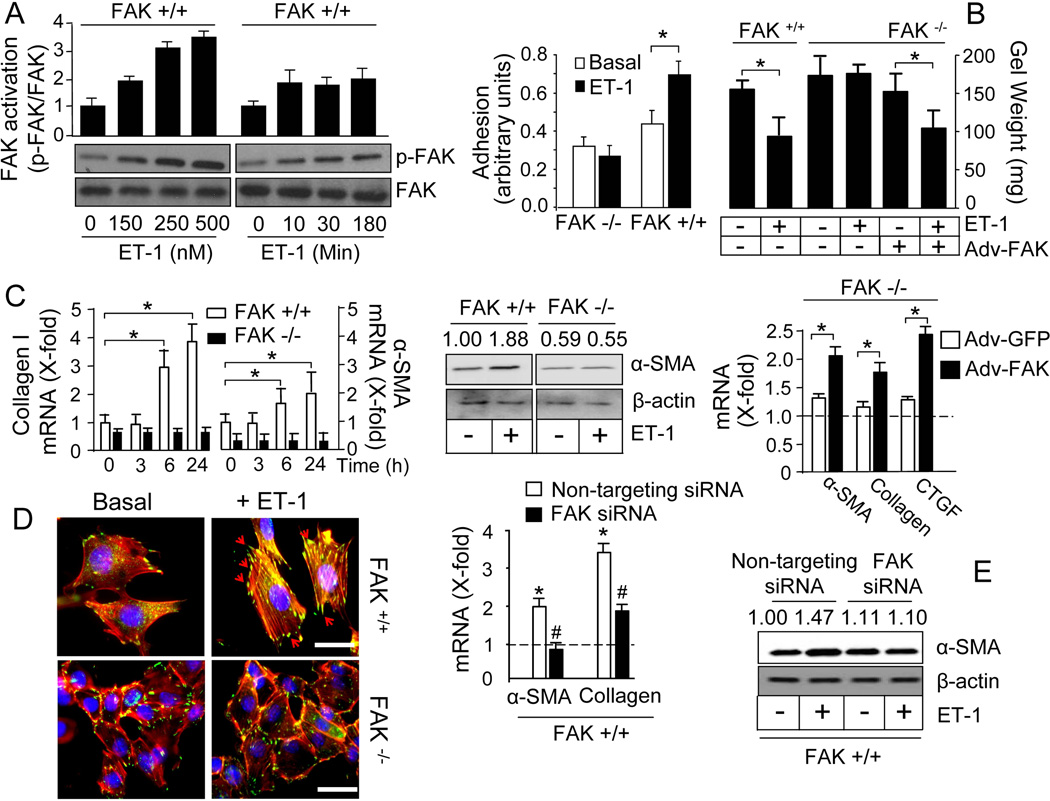
In order to analyze whether FAK is required for the acquisition of a profibrotic phenotype, we have performed experiments in fibroblasts isolated from wild type and FAK-deficient mouse embryos using ET-1 as stimulus, since ET-1 has been shown to be able to activate FAK and to induce fibrosis both in vitro and in vivo (15, 22, 30–34). ET-1 induced FAK phosphorylation in a time- and dose-dependent manner in wild type fibroblasts (Fig.5A). As expected, neither FAK nor p-FAK was detected in FAK-deficient fibroblasts (data not shown). Accordingly, ET-1 enhanced the capacity of the cells to adhere to fibronectin matrices in wild type, but not in FAK-deficient fibroblasts (Fig.5B). The ability of wild-type fibroblasts to contract a floating collagen gel lattice in response to ET-1 was impaired in FAK-deficient fibroblasts (Fig.5B). FAK was also required for ET-1-mediated induction of the expression of profibrotic genes collagen type I and α-SMA as assessed by real time quantitative PCR (RT-qPCR) and immunoblotting (Fig.5C). Restoring FAK expression with adenoviral-mediated overexpression constructs rescued ET-1-induced collagen gel contraction and ET-1-dependent capacity to induce the expression of profibrotic genes in FAK-deficient cells (Fig.5C). We further explored the actions of ET-1 by immunofluorescence analysis. Wild type fibroblasts responded to ET-1 with the formation of “supermature” vinculin-containing focal adhesions which co-localized with an organized network of stress fibers in the cell periphery (Fig.5D). In contrast, FAK-deficient fibroblasts failed to form functional focal adhesion complexes in the absence or presence of ET-1. In concordance with experiments in FAK-deficient fibroblasts, siRNA-mediated downregulation of FAK diminished the capacity of ET-1 to promote the expression of profibrotic genes α-SMA and collagen type I in wild type fibroblasts (Fig.5E). FAK siRNA effectively reduced FAK expression in wild type fibroblasts as assessed by immunoblotting (Suppl. Fig.2A).
Figure 5. Loss of FAK in fibroblasts impairs the ability of ET-1 to induce the profibrotic phenotype.
Experiments were done in fibroblasts from wild type and FAK-deficient mice as indicated. A) Time- and dose-dependent effect of ET-1 on p-FAK and FAK, n=3. B) Cell adhesion (left) and contraction (right) under basal conditions or after ET-1, n=4, *p <0.05. C) Analysis of profibrotic gene expression. Time course induction by ET-1 of collagen type I and α-SMA mRNA expression, n=3, *p <0.05 (left). Effect of ET-1 on α-SMA protein (middle, n=3). Induction by ET-1 of α-SMA, collagen type I and CTGF mRNA expression in FAK −/− fibroblasts transduced by adenoviral infection with FAK, or with GFP as a control, n=3, *p <0.05 (right). The dotted line represents the normalized value without ET-1. D) Immunolocalization of vinculin (green) and phalloidin staining of actin filaments (red) (scale bar: 50 µm, n=3). The arrows indicate the formation of vinculin-containing focal adhesions. E) Effect of siRNA-mediated knockdown of FAK expression on ET-1-induced α-SMA and collagen type I mRNA, *p <0.05 comparing ET-1-induced versus basal, #p<0.05 comparing FAK-specific versus non-targeting siRNA, (left, n=3); and α-SMA protein expression (right, n=3). Values above the blots refer to the ratio of the signals p-FAK/FAK or α-SMA/β-actin after densitometric analysis.
ET-1-dependent profibrotic gene expression is driven by Jun N-terminal kinase (JNK) via FAK
Our results in wild type and FAK-deficient fibroblasts indicate that ET-1 induces cell adhesion, matrix contraction, stress fiber formation, and profibrotic gene expression, all of these being features of myofibroblast differentiation, and that the loss of FAK impairs these actions. In fact, FAK is required for an entire matrix remodeling gene expression program, as shown in expression profile experiments (Suppl. Table I). ET-1 is able to induce the expression of a number of adhesion, extracellular matrix remodeling and cytoskeletal genes in wild type but not in FAK-deficient fibroblasts. These genes include integrin α1, several laminins, fibulin 5 and procollagen XI α1, among others. We also found a set of additional fibrosis-related genes that were also induced by ET-1 in a FAK-dependent manner. This group included insulin-like growth factor I receptor (IGFI receptor), IGF binding protein 7, platelet-derived growth factor D, Rho GTPase activating protein 24 and SRY-box containing gene 11 (SOX11) (35–39). Interestingly, ET-1 induced the expression of an additional set of genes both in wild type and FAK-deficient, for example early growth response 1 (Egr-1), indicating that FAK-independent signaling pathways are still active in FAK-deficient fibroblasts.
FAK associates with several different signaling proteins such as the Src family of protein tyrosine kinases, p130Cas, Shc, Grb2, PI3 kinase, and paxillin. This enables FAK to function within a network of integrin-stimulated signaling pathways leading to the activation of targets such as the ERK and JNK/mitogen-activated protein kinases. These pathways in turn transmit signals to the nucleus where a profibrotic expressional program is initiated. We have previously described that JNK activation in fibrotic lung fibroblasts contributes to the persistence of the myofibroblast phenotype by promoting an autocrine ET-1 loop, and other authors have showed that JNK is required for the development of pulmonary fibrosis (40, 41). We therefore asked whether FAK was required for constitutive ET-1-dependent JNK activation. ET-1 induced JNK phosphorylation in wild type but not in FAK-deficient fibroblasts (Suppl. Fig.3A,B). JNK is indeed downstream of FAK in the signal transduction pathway as shown in experiments performed with the novel inhibitor of FAK, PF-562,271, with the Src inhibitor, PP2, and with the JNK inhibitor, SP600125. PF-562,271 and PP2 inhibited both FAK and JNK activation by ET-1; SP600125 impaired only JNK phosphorylation without any significant modification of FAK activation (Suppl. Fig.3C,D). As a more specific tool for the inhibition of JNK, we promoted the overexpression of a dominant-negative form of JNK. Suppl. Fig.3E–G also shows that this form prevented the ET-1-induced activation of endogenous JNK, as well as the effect of ET-1 on α–SMA and collagen expression, without altering FAK activation.
ET-1-induced FAK phosphorylation is dependent on cell adhesion and integrin β1 signaling
FAK occupies a central position in the environment-sensing cell system as it integrates signals coming from both the extracellular matrix via integrins and from growth factors acting on specific receptors (13). In this regard, we have recently reported that activation of integrin β1-FAK axis by the adhesion of fibroblasts to matrix contributes to the induction of pro-fibrotic gene expression (31). Based on the finding that ET-1 signals through FAK in fibroblasts, we sought to analyze whether integrin-mediated cell adhesion to matrix and ET-1 signaling could cooperate in the regulation of FAK activity and subsequent fibroblast fate. We seeded primary mouse lung fibroblasts on culture plates treated or not with poly-lysine (poly-Lys), as well as employed cells left in suspension. Cells on untreated culture plates, which adhere mainly in an integrin-dependent way through serum-derived fibronectin, displayed a basal FAK activity that was further increased by ET-1 treatment (42). Cells on poly-Lys showed reduced FAK phosphorylation that could not be elevated by ET-1 treatment. Suspended cells showed no detectable FAK activity (Fig.6A). As integrin signaling is impaired in poly-Lys-coated matrices or in cells in suspension, these results indicate that integrin-mediated signal activation is required for ET-1 to induce FAK activity. The activation of FAK showed a correlation with the induction of the myofibroblast marker, α-SMA. ET-1-induced α-SMA was only observed in cells seeded on plastic, but not in those seeded on poly-Lys or suspended cells (Fig.6B). FAK interacts with the cytoplasmic tail of integrin β1 subunits (43, 44). In order to test the specific contribution of integrin β1 we obtained fibroblasts from wild type and integrin β1-deficient mice. ET-1 was able to induce FAK in wild type fibroblasts but not in integrin β1-deficient cells (Fig.6C). Accordingly, α-SMA induction by ET-1 was impaired in integrin β1-deficient cells (Fig.6D). In conclusion, these results indicate that cell adhesion through integrin β1 is required for ET-1 to promote the activation of FAK and the induction of a profibrotic expression program.
Figure 6. Induction of FAK phosphorylation by ET-1 is dependent on cell adhesion and integrin β1 signaling.
Analysis of FAK phosphorylation (A) and α-SMA protein expression (B) in fibroblasts seeded on standard tissue culture plates (Plastic), culture plates coated with 10 µg/ml poly-lysine (Poly-Lys), and cells left in suspension (Suspended). Detection of p-FAK (C) and α-SMA protein expression (D) in fibroblasts from wild type (ITGβ1 +/+) and integrin β1-deficient (ITGβ1 −/−) mice seeded on standard culture plates. Blots and histograms depicting the ratio p-FAK/FAK show the result of a representative experiment. Values above the blots refer to the ratio of the signal p-FAK/FAK or α-SMA/β-actin after densitometric analysis, n=3.
Discussion
Fibrosis is a recognized feature of many chronic lung diseases and is central to the pathogenesis of pulmonary fibrosis. The hallmark lesions of the disease are fibroblast foci, which are sites featuring activated myofibroblasts leading to the exaggerated accumulation of extracellular matrix with the irreversible destruction of lung parenchyma. Although the understanding of the pathogenesis of pulmonary fibrosis has increased over the last years, an efficacious treatment remains elusive. Therefore, the search and identification of specific targets for therapeutic intervention is essential for the development of new drugs. In the present article we provide evidence for the contribution of FAK to the signal transduction pathways leading to fibrosis in vivo and in vitro.
Previous evidence has already shown enhanced FAK activity in fibroblasts from IPF lungs (19). Here we extend these observations by performing a more comprehensive comparison of FAK activity and expression in lungs from pulmonary fibrosis patients (CTD-PF and IPF) and healthy donors by immunohistochemistry. Our studies show that a significant number of α-SMA-expressing myofibroblasts within fibrotic foci in pulmonary fibrosis patients were positive for FAK expression and activity. Highly expressed FAK was also found in bronchial epithelial cells both in fibrotic and normal lungs, where it may be involved in epithelial layer integrity and survival (45). In fact, the presence of upregulated FAK in bronchial epithelial cells may be indicative that lung epithelial cells contribute to the generation of myofibroblasts through a process of EMT in a FAK-dependent manner (46, 47). Nevertheless, the full impact and significance of this process for lung fibrogenesis and disease progression still needs to be defined. Our immunohistochemical analysis also detected strong FAK activity in smooth muscle and endothelial layers within remodeled lung vasculature in pulmonary fibrosis patients, where we found a highly significant association between the thickening of the media layer and the extent of FAK activation. In fact, intimal hyperplasia resulting from proliferation and migration of vascular smooth muscle cells requires an intact FAK activity as recently demonstrated with the overexpression of a dominant negative form of FAK, FAK-related non-kinase (48). Additionally, damage to endothelial cells may also contribute to the formation of myofibroblasts with the involvement of FAK, as described for oxidative stress-challenged mouse endothelial cells (49). Therefore, it seems likely that the vascular remodeling and pulmonary hypertension associated to the progression of lung fibrosis may also imply the activation of FAK. Nevertheless, this particular point was not further investigated as the bleomycin-induced lung fibrosis mouse model used in our studies is not suitable for the analysis of the chronic vascular changes associated to IPF.
Fibrosis is often defined as a wound healing response that has gone beyond control and in which the repair phase results in deleterious changes. In response to tissue injury, a set of tightly orchestrated repertoire of cellular responses is triggered favoring the generation of myofibroblasts, which are considered proficient cells for the repair process of the wound. Wherever the myofibroblasts arise, either from epithelial or endothelial cells undergoing EMT (or endoMT) or from resident or circulating fibroblasts, these cells acquire during the differentiation process the ability to attach through focal adhesions to the fibronectin deposited in the early phases of tissue repair, with concomitant activation of FAK-dependent pathways. Cell adhesion is not merely a process engaging attachment of fibroblasts to ECM but also promoting downstream expression of genes involved in tissue remodeling and repair, as recently described by our group (31). In fact, persistently activated adhesion and adhesive signaling, including FAK activation, is a hallmark of fibrotic cells (20). Using wild type and FAK-deficient fibroblasts and ET-1 as stimulus we proved in this study that FAK is required for ET-1 to induce a profibrotic phenotype. FAK activation is transmitted through JNK to promote the induction of the expression of profibrotic genes involved in enhanced adhesion, migration, contraction and myofibroblast differentiation. Our results also endorse that FAK activation by ET-1 requires ECM-mediated signaling through integrins, particularly integrin β1, as it was impaired in cells seeded on poly-Lys or kept in suspension, and in integrin β1-deficient fibroblasts. Thus, FAK serves as a converging hub in the signaling pathway triggered by ECM via integrins and growth factor receptors. Besides ET-1, other cytokines/mediators believed to be implicated in the pathogenesis of lung fibrosis, such as TGF-β1 or CTGF, have been also reported to signal through FAK (14–17, 31). These reports together with our observations highlight FAK as a “common signaling node” in myofibroblast formation and behavior, and support that FAK inhibition may represent a novel anti-fibrotic therapeutic approach. To this respect, therapeutic approaches based on the inhibition of one target have failed to protect against IPF progression, for example, the blockade of ET receptors with specific antagonists, as shown recently in BUILD-3 trial of bosentan (www.actelion.com) or further highlighted by Gilead’s recent decision to stops ARTEMIS-IPF trial of ambrisentan, due to lack of efficacy (www.gilead.com). The results of these clinical trials may indicate that the inhibition of just one of the upstream activators of FAK (e.g. ET-1) may not be sufficient for the achievement of therapeutic objectives. In order to validate FAK as a potential target, we tested the effect of reducing FAK activity and expression with the novel pharmacological inhibitor of FAK, PF-562,271, and by siRNA-mediated knockdown, respectively. Both treatments attenuated the extent of fibrotic response in the in vivo model of bleomycin-induced lung fibrosis as assessed by histological and immunohistochemical methodologies. Oral FAK inhibitors such as PF-562,271 initially developed as anticancer drugs may therefore prove to be useful in the treatment of fibrotic diseases. To date, no weight loss, morbidity, or mortality has been reported in in vivo experiments or in initial phase 1 clinical trials, demonstrating that PF-562,271 is safe and well-tolerated (27, 28, 50). Our results suggest that this drug, which represents the sole FAK inhibitor being tested in humans to date, may offer therapeutic power for the treatment of fibrosis-related ailments.
In conclusion, we report here that FAK functions as a central mediator of fibrogenesis, acting as a key point of convergence integrating cell matrix-adhesion and ET signaling. Our study also purports the notion that FAK inhibition may represent a useful therapeutic tool in the treatment of fibrotic diseases.
Supplementary Material
Acknowledgments
Funded by:
Ministerio de Ciencia e Innovación, Plan Nacional de I+D+I (SAF2006-02410, SAF2009-09085, SAF2009-07520 and Consolider CSD-2007-0020: ROSASNET).
Comunidad Autónoma de Madrid (CARDIOVREP Consortium).
Sociedad Española de Nefrología (Senefro grant).
Fundación Médica Mutua Madrileña (FundacionMM grant).
Fundación Genoma España (Meica project).
Canadian Institute of Health Research.
Canadian Foundation for Innovation.
Ontario Thoracic Society.
National Institutes of Health (NIH R01-HL095732 and NIH K08-HL105656).
References
- 1.Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE, Jr, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142(12 Pt 1):963–967. doi: 10.7326/0003-4819-142-12_part_1-200506210-00005. [DOI] [PubMed] [Google Scholar]
- 3.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117(3):557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117(3):524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 6.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 7.Shi-Wen X, Renzoni EA, Kennedy L, Howat S, Chen Y, Pearson JD, et al. Endogenous endothelin-1 signaling contributes to type I collagen and CCN2 overexpression in fibrotic fibroblasts. Matrix Biol. 2007;26(8):625–632. doi: 10.1016/j.matbio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Xu SW, Blumbach K, Eastwood M, Denton CP, Eckes B, et al. Expression of integrin beta1 by fibroblasts is required for tissue repair in vivo. J Cell Sci. 2010;123(Pt 21):3674–3682. doi: 10.1242/jcs.070672. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Kapoor M, Denton CP, Abraham DJ, Leask A. Loss of beta1 integrin in mouse fibroblasts results in resistance to skin scleroderma in a mouse model. Arthritis Rheum. 2009;60(9):2817–2821. doi: 10.1002/art.24801. [DOI] [PubMed] [Google Scholar]
- 11.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, et al. Epithelial cell alpha3-beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. The Journal of Clinical Investigation. 2009;119(1):213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323(5914):642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 13.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2(5):249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 14.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278(14):12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 15.Daher Z, Noel J, Claing A. Endothelin-1 promotes migration of endothelial cells through the activation of ARF6 and the regulation of FAK activity. Cell Signal. 2008;20(12):2256–2265. doi: 10.1016/j.cellsig.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Xu SW, Kennedy L, Pala D, Chen Y, Eastwood M, et al. FAK is required for TGFbeta-induced JNK phosphorylation in fibroblasts: implications for acquisition of a matrix-remodeling phenotype. Mol Biol Cell. 2007;18(6):2169–2178. doi: 10.1091/mbc.E06-12-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan TW, Lai CH, Huang CY, Yang WH, Chen HT, Hsu HC, et al. CTGF enhances migration and MMP-13 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells. J Cell Biochem. 2009;107(2):345–356. doi: 10.1002/jcb.22132. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira A, Chaverot N, Schroder C, Strosberg AD, Couraud PO, Cazaubon S. Requirement of caveolae microdomains in extracellular signal-regulated kinase and focal adhesion kinase activation induced by endothelin-1 in primary astrocytes. J Neurochem. 1999;72(1):120–128. doi: 10.1046/j.1471-4159.1999.0720120.x. [DOI] [PubMed] [Google Scholar]
- 19.Cai GQ, Zheng A, Tang Q, White ES, Chou CF, Gladson CL, et al. Downregulation of FAK-related non-kinase mediates the migratory phenotype of human fibrotic lung fibroblasts. Exp Cell Res. 2010;316(9):1600–1609. doi: 10.1016/j.yexcr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mimura Y, Ihn H, Jinnin M, Asano Y, Yamane K, Tamaki K. Constitutive Phosphorylation of Focal Adhesion Kinase Is Involved in the Myofibroblast Differentiation of Scleroderma Fibroblasts. J Investig Dermatol. 2005;124(5):886–892. doi: 10.1111/j.0022-202X.2005.23701.x. [DOI] [PubMed] [Google Scholar]
- 21.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14(1):45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 22.Lagares D, Garcia-Fernandez RA, Jimenez CL, Magan-Marchal N, Busnadiego O, Lamas S, et al. Endothelin 1 contributes to the effect of transforming growth factor beta1 on wound repair and skin fibrosis. Arthritis Rheum. 2010;62(3):878–889. doi: 10.1002/art.27307. [DOI] [PubMed] [Google Scholar]
- 23.Ding Q, Gladson CL, Wu H, Hayasaka H, Olman MA. Focal adhesion kinase (FAK)-related non-kinase inhibits myofibroblast differentiation through differential MAPK activation in a FAK-dependent manner. J Biol Chem. 2008;283(40):26839–26849. doi: 10.1074/jbc.M803645200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, et al. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166(2):367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corte TJ, Wort SJ, Wells AU. Pulmonary hypertension in idiopathic pulmonary fibrosis: a review. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26(1):7–19. [PubMed] [Google Scholar]
- 26.Bagi CM, Christensen J, Cohen DP, Roberts WG, Wilkie D, Swanson T, et al. Sunitinib and PF-562,271 (FAK/Pyk2 inhibitor) effectively block growth and recovery of human hepatocellular carcinoma in a rat xenograft model. Cancer Biol Ther. 2009;8(9):856–865. doi: 10.4161/cbt.8.9.8246. [DOI] [PubMed] [Google Scholar]
- 27.Bagi CM, Roberts GW, Andresen CJ. Dual focal adhesion kinase/Pyk2 inhibitor has positive effects on bone tumors: implications for bone metastases. Cancer. 2008;112(10):2313–2321. doi: 10.1002/cncr.23429. [DOI] [PubMed] [Google Scholar]
- 28.Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68(6):1935–1944. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 29.Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123(Pt 7):1007–1013. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- 30.Abraham DJ, Vancheeswaran R, Dashwood MR, Rajkumar VS, Pantelides P, Xu SW, et al. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am J Pathol. 1997;151(3):831–841. [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy L, Shi-Wen X, Carter DE, Abraham DJ, Leask A. Fibroblast adhesion results in the induction of a matrix remodeling gene expression program. Matrix Biol. 2008;27(4):274–281. doi: 10.1016/j.matbio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, et al. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15(6):2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi-Wen X, Denton CP, Dashwood MR, Holmes AM, Bou-Gharios G, Pearson JD, et al. Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. J Invest Dermatol. 2001;116(3):417–425. doi: 10.1046/j.1523-1747.2001.01256.x. [DOI] [PubMed] [Google Scholar]
- 34.Xu SW, Howat SL, Renzoni EA, Holmes A, Pearson JD, Dashwood MR, et al. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J Biol Chem. 2004;279(22):23098–23103. doi: 10.1074/jbc.M311430200. [DOI] [PubMed] [Google Scholar]
- 35.Skubitz KM, Skubitz AP. Gene expression in aggressive fibromatosis. J Lab Clin Med. 2004;143(2):89–98. doi: 10.1016/j.lab.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Ponten A, Folestad EB, Pietras K, Eriksson U. Platelet-derived growth factor D induces cardiac fibrosis and proliferation of vascular smooth muscle cells in heart-specific transgenic mice. Circ Res. 2005;97(10):1036–1045. doi: 10.1161/01.RES.0000190590.31545.d4. [DOI] [PubMed] [Google Scholar]
- 37.Pilewski JM, Liu L, Henry AC, Knauer AV, Feghali-Bostwick CA. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am J Pathol. 2005;166(2):399–407. doi: 10.1016/S0002-9440(10)62263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasuoka H, Zhou Z, Pilewski JM, Oury TD, Choi AM, Feghali-Bostwick CA. Insulin-like growth factor-binding protein-5 induces pulmonary fibrosis and triggers mononuclear cellular infiltration. Am J Pathol. 2006;169(5):1633–1642. doi: 10.2353/ajpath.2006.060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu E, Feghali-Bostwick CA. Insulin-like growth factor-II is increased in systemic sclerosis-associated pulmonary fibrosis and contributes to the fibrotic process via Jun N-terminal kinase- and phosphatidylinositol-3 kinase-dependent pathways. Am J Pathol. 2008;172(6):1580–1590. doi: 10.2353/ajpath.2008.071021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi-Wen X, Rodriguez-Pascual F, Lamas S, Holmes A, Howat S, Pearson JD, et al. Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol Cell Biol. 2006;26(14):5518–5527. doi: 10.1128/MCB.00625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alcorn JF, van der Velden J, Brown AL, McElhinney B, Irvin CG, Janssen-Heininger YM. c-Jun N-terminal kinase 1 is required for the development of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2009;40(4):422–432. doi: 10.1165/rcmb.2008-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Wachem PB, Mallens BW, Dekker A, Beugeling T, Feijen J, Bantjes A, et al. Adsorption of fibronectin derived from serum and from human endothelial cells onto tissue culture polystyrene. J Biomed Mater Res. 1987;21(11):1317–1327. doi: 10.1002/jbm.820211104. [DOI] [PubMed] [Google Scholar]
- 43.Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995;130(5):1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen LM, Bailey D, Fernandez-Valle C. Association of beta 1 integrin with focal adhesion kinase and paxillin in differentiating Schwann cells. J Neurosci. 2000;20(10):3776–3784. doi: 10.1523/JNEUROSCI.20-10-03776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garneau-Tsodikova S, Thannickal VJ. Protein kinase inhibitors in the treatment of pulmonary fibrosis. Curr Med Chem. 2008;15(25):2632–2640. doi: 10.2174/092986708785908969. [DOI] [PubMed] [Google Scholar]
- 46.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103(35):13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng B, Yang X, Liu J, He F, Zhu Z, Zhang C. Focal adhesion kinase mediates TGF-beta1-induced renal tubular epithelial-to-mesenchymal transition in vitro. Mol Cell Biochem. 2010;340(1–2):21–29. doi: 10.1007/s11010-010-0396-7. [DOI] [PubMed] [Google Scholar]
- 48.Brewster LP, Ucuzian AA, Brey EM, Liwanag M, Samarel AM, Greisler HP. FRNK overexpression limits the depth and frequency of vascular smooth muscle cell invasion in a three-dimensional fibrin matrix. J Cell Physiol. 2010;225(2):562–568. doi: 10.1002/jcp.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sen U, Moshal KS, Tyagi N, Kartha GK, Tyagi SC. Homocysteine-induced myofibroblast differentiation in mouse aortic endothelial cells. J Cell Physiol. 2006;209(3):767–774. doi: 10.1002/jcp.20752. [DOI] [PubMed] [Google Scholar]
- 50.Xu H, Yin D, Pierce KJ, Shreeve SM, Duncan B, Bello A. Pharmacokinetics (PK) of PF-562271, a focal adhesion kinase (FAK) inhibitor, and its effect on CYP3A in patients with advanced nonhemotologic malignancies. J Clin Oncol. 2010;28(15_suppl) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.