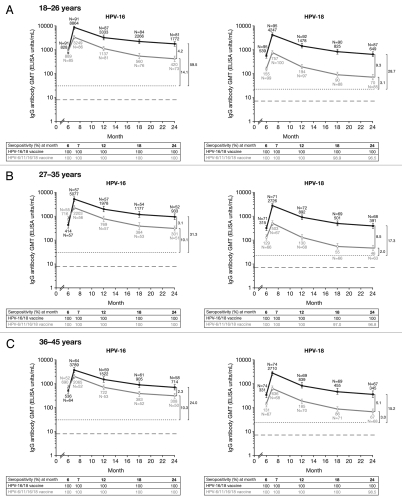
Figure 3.
Geometric mean titers (GMTs), Month 24 GMT ratios (GMR) and seropositivity rates for anti-HPV-16 and anti-HPV-18 IgG antibodies measured by enzyme-linked immunosorbent assay at Months 6, 7, 12, 18 and 24 (ATP cohort for immunogenicity, seronegative and DNA negative prior to vaccination). Solid black lines, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant adjuvanted, adsorbed) [Cervarix®]; solid gray lines, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant [Gardasil®]. Error bars denote 95% confidence intervals (CIs) of GMTs. Dotted line, GMTs for natural infection antibody levels (measured by ELISA) in the HPV-008 study: 29.8 ELISA units/mL for HPV-16 and 22.6 ELISA units/mL for HPV-18.10 GMTs for natural infection antibody levels (measured by ELISA) in the HPV-001 14 were 50 EU/mL for anti HPV-16 antibodies and 41 EU/mL anti HPV-18 antibodies. Vaccine induced antibody levels remained above the levels associated with natural infection in women in the ATP cohort for immunogenicity who received all three doses of HPV-16/18 vaccine through to 7.3 y follow-up of Study HPV-001 (HPV-007, HPV-023) 14,15,44 and through to Month 36 follow-up of Study HPV-008.10,13 Dashed line, ELISA limit of detection (8 ELISA units/mL for HPV-16 and 7 ELISA units/mL for HPV-18). Black square brackets, GMRs = HPV-16/18 vaccine GMT divided by HPV-6/11/16/18 vaccine GMT at Month 24 computed using an ANOVA model on the log10 transformation of the titers in each age cohort. Seropositivity defined as neutralizing antibody titer ≥8 ELISA units/mL for HPV-16 and ≥7 ELISA units for HPV-18. The ATP cohort for immunogenicity included all evaluable subjects who received three vaccine doses (i.e., those meeting all eligibility criteria and complying with the procedures defined in the protocol) for whom data concerning immunogenicity endpoint measures were available. This included subjects for whom assay results were available for antibodies against at least one study vaccine antigen (HPV-16 or HPV-18) at the timepoint under analysis.