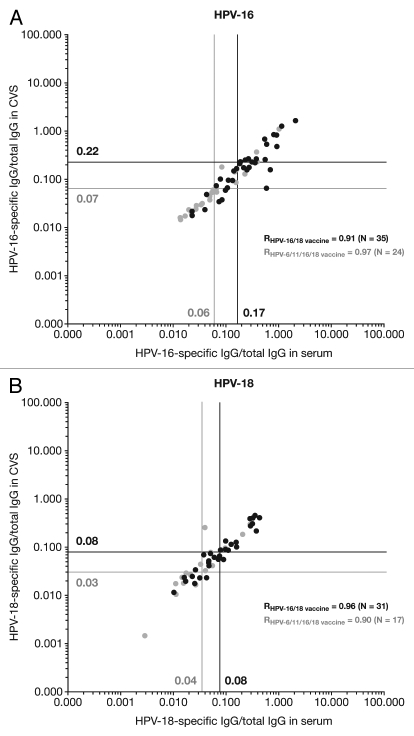
Figure 4.
Correlation between serum and cervicovaginal secretions for (A) HPV-16 and (B) HPV-18 IgG antibodies (standardized for total IgG antibodies) measured by enzyme-linked immunosorbent assay at Month 24 (ATP cohort for immunogenicity). CVS, cervicovaginal secretions; IgG, immunoglobulin G; R, Pearson correlation coefficient. Solid black lines and filled black circles, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) [Cervarix®]; solid gray lines and filled gray circles, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant [Gardasil®]. Only samples that tested positive for the antigen under analysis in both serum and CVS (i.e., double-positive samples) were analyzed. Vertical and horizontal lines represent the geometric means of the ratios between HPV-specific antibody titers and total IgG antibody content in serum and CVS samples, respectively. HPV-16/18-specific IgG levels were measured by VLP-specific ELISA. The total IgG antibody concentration of each sample was measured using an ELISA developed and validated in-house by GSK.