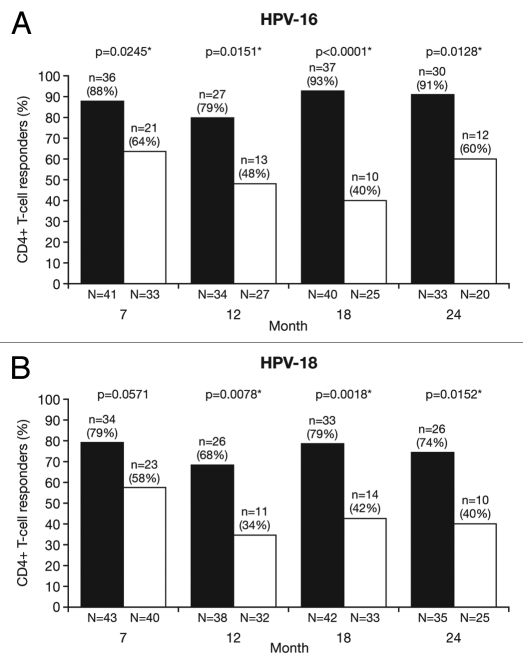
Figure 7.
Proportion of responders for (A) HPV-16- and (B) HPV-18-specific CD4+ T-cell response at Months 7, 12, 18 and 24 (ATP cohort for immunogenicity; seronegative, DNA-negative and with a HPV type-specific CD4+ T-cell response below 500 cells per million cells prior to vaccination). *p < 0.05. N, number of subjects with available results. Black bars, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); white bars, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). Responders defined as subjects with ≥500 HPV type-specific memory CD4+ T-cells expressing at least two of four immune markers [(CD40L, IL-2, TNFα, IFNγ)/million cells]. p-values were calculated using Fisher's exact test to compare proportion of responders.