Abstract
Background
Hypoglycemia can be a symptom in patients with Addison's disease. The common regimen of replacement therapy with oral glucocorticoids results in unphysiological low cortisol levels in the early morning, the time of highest insulin sensitivity. Therefore patients with Addison's disease are at risk for unrecognized and potentially severe nocturnal hypoglycemia also because of a disturbed counterregulatory function. Use of a continuous glucose monitoring system (CGMS) could help to adjust hydrocortisone treatment and to avoid nocturnal hypoglycemia in these patients.
Methods
Thirteen patients with Addison's disease were screened for hypoglycemia wearing a CGMS for 3–5 days.
Results
In one patient we identified a hypoglycemic episode at 3:45 a.m. with a blood glucose level of 46 mg/dL, clearly beneath the 95% tolerance interval of minimal glucose levels between 2 and 4 a.m. (53.84 mg/dL). After the hydrocortisone replacement scheme was changed, the minimum blood glucose level between 2 and 4 a.m. normalized to 87 mg/dL.
Conclusions
Continuous glucose monitoring can detect nocturnal hypoglycemia in patients with primary adrenal insufficiency and hence prevent in these patients an impaired quality of life and even serious adverse effects.
Background
Hypoglycemia can be a symptom in patients with so far undiagnosed and untreated primary adrenal insufficiency (Addison's disease) but also in diagnosed patients with an unsatisfactory treatment. The lack of glucocorticoids increases glucose oxidation and decreases endogenous glucose production, leading to an increased insulin sensitivity.1 Storage of glucagon is inhibited. Patients with Addison's disease often suffer from symptoms reminiscent of neuroglycopenia, suggesting that this disorder is associated with a deficit in cerebral energy supply.2 However, there are few data about hypoglycemia, particularly nocturnal, in patients with Addisons's disease.
Common glucocorticoid replacement is oral hydrocortisone split into two to three daily doses, with the highest dose administered in the morning.3,4 Whereas cortisol in healthy individuals rises abruptly in the early morning between 3 and 6 a.m., this physiological biorhythm cannot be achieved with the oral glucocorticoids currently available.
Due to the physiological biorhythm of other hormones involved in glucose metabolism—growth hormone in particular—insulin sensitivity is normally highest in the early morning hours between 2 and 4 a.m. Patients with diabetes receiving insulin treatment are at risk particularly for nocturnal hypoglycemia at exactly that time.
Patients with adrenal insufficiency receiving common oral treatment with hydrocortisone experience unphysiological low cortisol levels in this susceptible period of time. Therefore these patients are at risk for nocturnal hypoglycemia. Potentially, hypoglycemia episodes are more severe and of higher risk in patients with Addison's disease because of the deficient counterregulatory excretion of cortisol. Symptoms of hypoglycemia can be lessened, and so hypoglycemia unawareness can be a problem in these patients.
Continuous glucose monitoring systems (CGMSs) are improved instruments of blood glucose control developed for patients with insulin-dependent diabetes mellitus with often-unrecognized hypoglycemia episodes.5–8
In patients with Addison's disease, a CGMS could aid to adjust hydrocortisone treatment and to avoid—potentially severe—nocturnal hypoglycemia.
Patients and Methods
Thirteen patients with Addison's disease (diagnosed as spontaneous primary adrenal insufficiency by low basal cortisol and after tetracosactide [Synacthen®, Novartis, Bagsvaerd, Denmark] stimulation as well as elevated aderenocorticotropin level) from our outpatient clinic were recruited for this study during routine clinical visits or by written information between October 2008 and April 2011. All patients living in the nearer catchment area of our hospital were invited to take part. Diagnosis of diabetes mellitus type 1 or type 2 and known impaired glucose tolerance were exclusion criteria. There were five male and eight female subjects. The mean age of the patients was 46 years (range, 21–71 years). The patients had a mean duration of Addison's disease of 13 years (range, 0.25–52 years).
The subjects were screened for hypoglycemia wearing a CGMS for 3–5 days. The system used was the Minimed® (Medtronic, Northridge, CA) subcutaneous CGMS® System Gold™. This system continuously measures subcutaneous tissue interstitial glucose levels, recording values on average every 5 min within a range of 40–400 mg/dL. It allows 288 measurements of glucose in a 24-h period. A monitor collects and stores the glucose data until they are downloaded into the com-station, where glucose values are displayed.
Sensors were applied by a certified diabetes nurse in our outpatient clinic. Patients were educated in using the sensor and calibration with blood glucose self-measurements three to four times per day. Calibration of the sensor was performed according to the protocol established. At the completion of the measuring period, the system was returned, and the data were downloaded to determine glucose patterns.
Normal distribution of minimum blood glucose values between 2 and 4 a.m. was proved by the Kolmogoroff–Smirnoff test, and the 95% parametric tolerance interval was determined.
Results
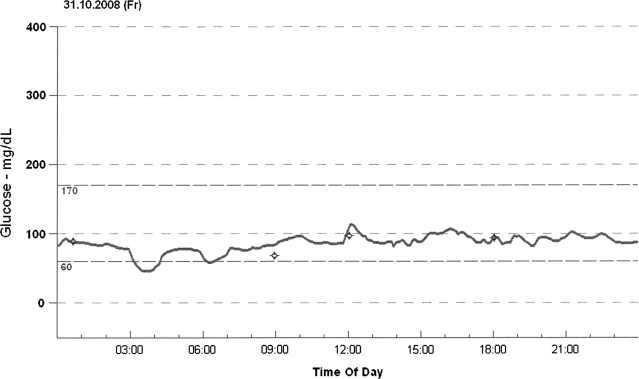
In one of the 13 patients we identified an hypoglycemia episode, defined by a blood glucose level of ≤50 mg/dL. The affected patient was a 42-year-old man with a history of Addison's disease for 33 years. Hypoglycemia was recorded at 3:45 a.m. with a blood glucose level of 46 mg/dL (Fig. 1). This value clearly falls below the lower limit of 95% tolerance interval of minimal glucose levels between 2 and 4 a.m. in the other patients, which was 53.84 mg/dL. The patient had been reported to wake up frequently at night for several months, but he had no typical signs of hypoglycemia. After his hydrocortisone replacement scheme was changed—he delayed the last hydrocortisone dose to the late evening—a second CGMS measurement was performed. No hypoglycemia was recorded, the minimum blood glucose level between 2 and 4 a.m. normalized to 87 mg/dL, and the patient has been observed to sleep better since that time.
FIG. 1.
Course of blood glucose levels in one patient with nocturnal hypoglycemia.
With regard to the whole measuring period, this patient showed lower glucose levels with a mean glucose level of 85 mg/dL compared with 99±12 mg/dL in the other patients and more (seven vs. one) and longer (9% vs. 0.5% of the measuring period) episodes with rather low glucose levels <70 mg/dL (Table 1).
Table 1.
Continuous Glucose Monitoring System Data in 13 Patients Without Diabetes With Addison's Disease
| Affected patients | All patients | |
|---|---|---|
| Mean glucose level (mg/dL) | 85 | 99±12 |
| Low excursions <70 mg/dL | ||
| Number | 7 | 1 (0–11) |
| % of measuring period | 9 | 0.5 (0–8) |
| Mean value of minimal glucose levels between 2 and 4 a.m. (mg/dL) | 46 | 77.6±12.71 |
Conclusions
Despite the substitution with synthetic gluco- and mineralocorticoids, premature mortality is still documented in Addison's disease.9,10 The absence of any valid laboratory parameter to assess adequate substitution effects continues to be one of the biggest challenges in the therapy of adrenal-insufficient patients.3,11 Herein an important problem is the unphysiological dosing with orally available glucocorticoids, particularly in the early morning hours when patients have a window of low cortisol levels—a fact that can lead to hypoglycemia at this time.
The mean glucose level in our patients with primary adrenal insufficiency was 99±12 mg/dL and therefore without any difference from the mean glucose level that has been seen in healthy controls using a CGMS.12 So, data of this small study do not allow us to conclude about glucose metabolism in adrenal-insufficient patients who are receiving chronic replacement—either for hypoglycemia episodes due to chronic cortisol deficiency or for hyperglycemia episodes due to chronic overtreatment.
However, in one individual with Addison's disease we could detect nocturnal hypoglycemia using the CGMS. Shifting the last hydrocortisone dose to the late evening normalized nocturnal blood glucose levels and improved his sleep.
Therefore continuous glucose monitoring can detect nocturnal hypoglycemia in patients with primary adrenal insufficiency and hence prevent impaired quality of life and potentially even serious adverse effects.
Further research on larger groups of patients is necessary to evaluate the risk of nocturnal hypoglycemia in primary adrenal insufficiency. New therapeutic approaches have been developed like continuous hydrocortisone infusion with hourly adjustable infusion rate via a subcutaneous pump13 or retarded hydrocortisone formulations given in the late evening.14 It will be of interest to learn whether these concepts affect nocturnal blood glucose levels.
Acknowledgments
This research was supported by grants from the EU FP7 (grant number 201167, Euradrenal).
Author Disclosure Statement
The authors have no competitive financial interests to disclose.
References
- 1.Christiansen JJ. Djurhuus CB. Gravholt CH. Iversen P. Christiansen JS. Schmitz O. Weeke J. Jorgensen JOL. Møller N. Effects of cortisol on carbohydrate, lipid, and protein metabolism: studies of acute cortisol withdrawal in adrenocortical failure. J Clin Endocrinol Metab. 2007;92:3553–3559. doi: 10.1210/jc.2007-0445. [DOI] [PubMed] [Google Scholar]
- 2.Klement J. Hubold C. Cords H. Oltmanns KM. Hallschmid M. Born J. Lehnert H. Peters A. High-calorie glucose-rich food attenuates neuroglycopenic symptoms in patients with Addison's disease. J Clin Endocrinol Metab. 2010;95:522–528. doi: 10.1210/jc.2009-1752. [DOI] [PubMed] [Google Scholar]
- 3.Mah PM. Jenkins RC. Rostami-Hodjegan A. Newell-Price J. Doane A. Ibbotson V. Tucker GT. Ross RJ. Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clin Endocrinol. 2004;61:367–375. doi: 10.1111/j.1365-2265.2004.02106.x. [DOI] [PubMed] [Google Scholar]
- 4.Crown A. Lightman S. Why is the management of glucocorticoid deficiency still controversial: a review of the literature. Clin Endocrinol. 2005;63:483–492. doi: 10.1111/j.1365-2265.2005.02320.x. [DOI] [PubMed] [Google Scholar]
- 5.Woodward A. Weston P. Casson IF. Gill GV. Nocturnal hypoglycaemia in type 1 diabetes—frequency and predictive factors. Q J Med. 2009;102:603–607. doi: 10.1093/qjmed/hcp082. [DOI] [PubMed] [Google Scholar]
- 6.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Validation of measures of satisfaction with and impact of continuous and conventional glucose monitoring. Diabetes Technol Ther. 2010;12:679–684. doi: 10.1089/dia.2010.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bode BW. Battelino T. Continous glucose monitoring. Int J Clin Pract Suppl. 2010;166:11–15. doi: 10.1111/j.1742-1241.2009.02272.x. [DOI] [PubMed] [Google Scholar]
- 8.Gross TM. Bode BW. Einhorn D. Kayne DM. Reed JH. White NH. Mastrototaro JJ. Performance evaluation of the MiniMed continuous glucose monitoring system during patient home use. Diabetes Technol Ther. 2000;2:49–56. doi: 10.1089/152091500316737. [DOI] [PubMed] [Google Scholar]
- 9.Hahner S. Loeffler M. Fassnacht M. Weismann D. Koschker AC. Quinkler M. Decker O. Arlt W. Allolio B. Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J Clin Endocrinol Metab. 2007;92:3912–3922. doi: 10.1210/jc.2007-0685. [DOI] [PubMed] [Google Scholar]
- 10.Bergthorsdottir R. Leonsson-Zachrisson M. Oden A. Johansson G. Premature mortality in patients with Addison's disease: a population-based study. J Clin Endocrinol Metab. 2006;91:4849–4853. doi: 10.1210/jc.2006-0076. [DOI] [PubMed] [Google Scholar]
- 11.Arlt W. Rosenthal C. Hahner S. Allolio B. Quality of glucocorticoid replacement in adrenal insufficiency: clinical assessment vs. timed serum cortisol measurements. Clin Endocrinol. 2006;64:384–389. doi: 10.1111/j.1365-2265.2006.02473.x. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez LM. Knight RJ. Heptulla RA. Continous glucose monitoring in subjects after simultaneous pancreas-kidney and kidney-alone transplantation. Diabetes Technol Ther. 2010;12:347–351. doi: 10.1089/dia.2009.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovas K. Husebye ES. Continous subcutaneous hydrocortisone infusion in Addison's disease. Eur J Endocrinol. 2007;157:109–112. doi: 10.1530/EJE-07-0052. [DOI] [PubMed] [Google Scholar]
- 14.Johansson G. Bergthorsdottir R. Nilsson AG. Lennernas H. Hedner T. Skrtic S. Improving glucocorticoid replacement therapy using a novel modified-release hydrocortisone tablet: a pharmacokinetic study. Eur J Endocrinol. 2009;161:119–130. doi: 10.1530/EJE-09-0170. [DOI] [PubMed] [Google Scholar]