Abstract
Objective
To demonstrate a cost-effective, portable, and simple-to-use fundus imaging system for laboratory animals.
Animals Studied
Albino rats, pigmented mice, albino guinea pigs, and New Zealand white rabbits.
Procedure
A contact fundus imaging system was designed and constructed using standard optical and mechanical components: a digital camera, an otoscope, a fiber optic light source, and standard optical lenses and mounts. Digital fundus video and photography of two albino rats, two pigmented mice, two New Zealand white rabbits, and two albino guinea pigs were obtained. For all animals examined, pupils were dilated and local anesthetic was administered.
Results
Digital images of the fundus were obtained in all animals. Contrast of retinal vasculature and overall image quality varied from one species to another, as the axial length, ocular optics, and retinal reflectance varied significantly across species. Light intensity and focus were optimized via the light source and lens focusing mount to produce high-quality images for each animal.
Conclusions
The portable, cost-effective contact fundus imaging system was easy to use for fundus examination of laboratory animals.
Keywords: endoscope, funduscopy, gradient-index, otoscope
INTRODUCTION
Fundus imaging is an established modality for screening and monitoring retinal diseases both in humans and in animal models. Funduscopy is used routinely to detect and evaluate retinal detachment or other eye diseases including glaucoma, age-related macular degeneration, and diabetic retinopathy. In animal models, funduscopy can be used to characterize the natural progression of a disease and the therapeutic effects of potential treatments as well. In particular, because of a rapidly growing ability to study gene function and to target genes in mice, funduscopy in mice is finding increasing interest.1
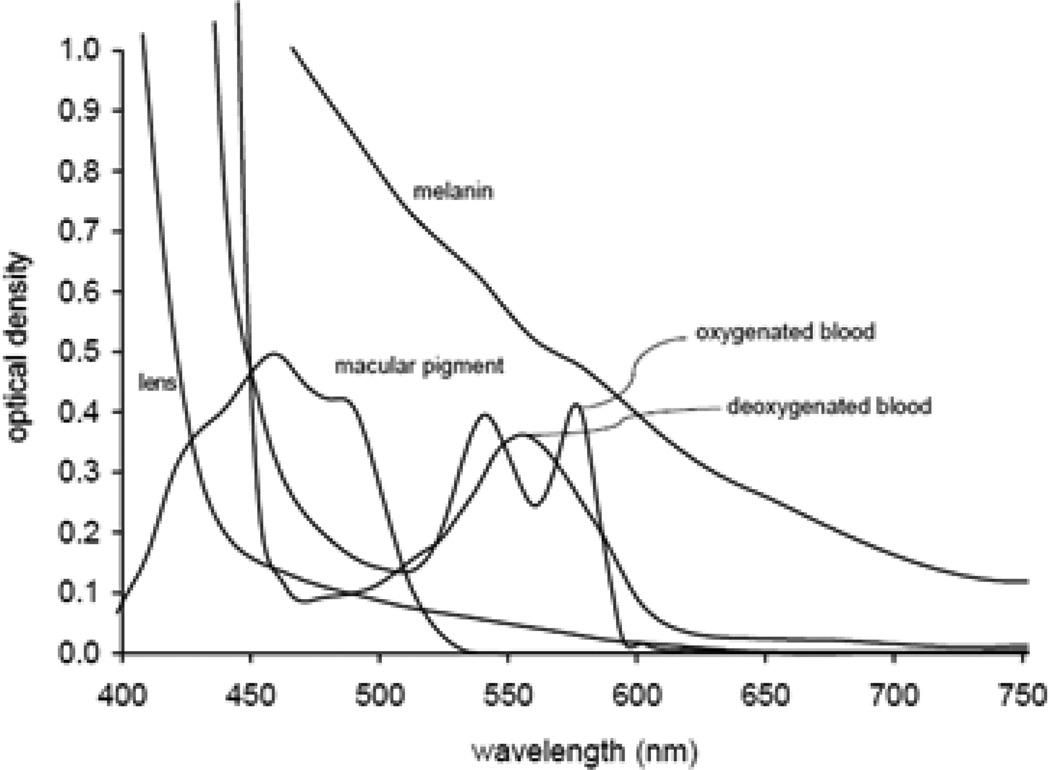
Until recently, fundus photography of mice has been complicated by the small size of the mouse eye and their very small pupil, which limits the amount of light reflected back from the fundus.2–4 Fundus reflectance has been an area of great interest in fundus imaging since the development of the first ophthalmoscopes in the 1850s. There are multiple factors affecting fundus reflectance, both directly in the light’s transpupillary path to the retina and peripheral to this path. The greatest amount of reflectance occurs at the cornea and lens owing to the large differences in refractive index between interfaces. These optical interfaces attenuate light approaching the fundus, limiting incident intensity, and therefore the amount of light that can be reflected from the fundus. A significant amount of light is also absorbed by ocular structures (Fig. 1). Reflectance and absorption contribute to overall fundus image quality, which consequently affects diagnosis of diseases or abnormalities.5
Figure 1.
Absorption spectrum of relevant components of the retina.5
Typically, images of the ocular fundus of animals are obtained by using a modified fundus camera, indirect ophthalmoscopy, confocal scanning laser ophthalmoscopy (cSLO), or optical coherence tomography, all of which are noncontact methods that relay the fundus image several centimeters in front of the cornea.6 However, these instruments are costly, bulky, and difficult to use in animal rooms, especially those in quarantine. Handheld fundus cameras like the Genesis (Kowa, Tokyo, Japan) are less bulky but remain costly and difficult to align with the animal eye.7 More recently, a new retinal camera especially designed to examine mice and rats was introduced but its prohibitive cost makes it difficult to justify in studies performed in biohazard rooms where highly contagious diseases such as tuberculosis are studied.
In the mid-1990s, French physicist Pascal Oliver Rol introduced the application of Gradient-Index (GRIN) lens technology for intraocular videophotography.8 GRIN lenses permit the transfer of images through a small glass rod and are widely used in the fabrication of endoscopes. Rol later developed a portable contact GRIN video fundus camera for retinal examination of babies suspected of having retinitis of prematurity. As the axial length of premature eyes is smaller than adults (16–18 vs. 24 mm), a first trial was performed in rabbits (axial length approximately 16–18 mm) at the Bascom Palmer Eye Institute’s Ophthalmic Biophysics Center in 2000. The work of Dr. Rol was published posthumously by his doctoral student and his colleagues. Later, a new contact fundus imaging technique using a GRIN endoscope was demonstrated in mice9 and later in larger animals including nonhuman primates.10
Contact endoscopic imaging provides high-resolution in vivo images of the fundus, ciliary body, and iridocorneal angle in a variety of animal species. The physical design of the system is based on the use of an endoscope with parallel illumination coupled to a digital camera. Although to date, this type of system has found limited use in research;11 the applicability of the system is likely to find increasing applications in the future.12–14 In this paper, we describe variations to the contact endoscopic fundus imaging system, which are successfully demonstrated in mice, rats, guinea pigs, and rabbits.
MATERIALS AND METHODS
Just as in previous endoscopic fundus imaging systems, the physical design of this system revolved around the use of a high-resolution digital camera and an endoscope. Our system design consists of:
12.1 mega pixel camera (D5000; Nikon, Tokyo, Japan).
Plano-concave lens (30 mm dia., −50.8 mm EFL, #12.002, Rolyn Optics, Covina, CA).
Video otoscope (#1328/0; Fiegert Endotech, Tuttlingen Germany).
Lens focusing mount (LFM-1A; Newport, Mountain View CA).
Two camera lens filters (NINC62; Nikon, Tokyo Japan).
Wireless camera remote control (NIML-L3; Nikon).
Xenon arc fiber optic light source (#611; Karl Storz, Tuttlingen Germany).
Fiber light guide (EC-1; Sunoptic Technologies, Jacksonville FL).
Engineering design (Fig. 2) was performed using CAD software (Solid Works 9, Concord, MA).
Figure 2.
Computer-Aided Design of the optical section of the Rol gradient-index fundus imaging system.
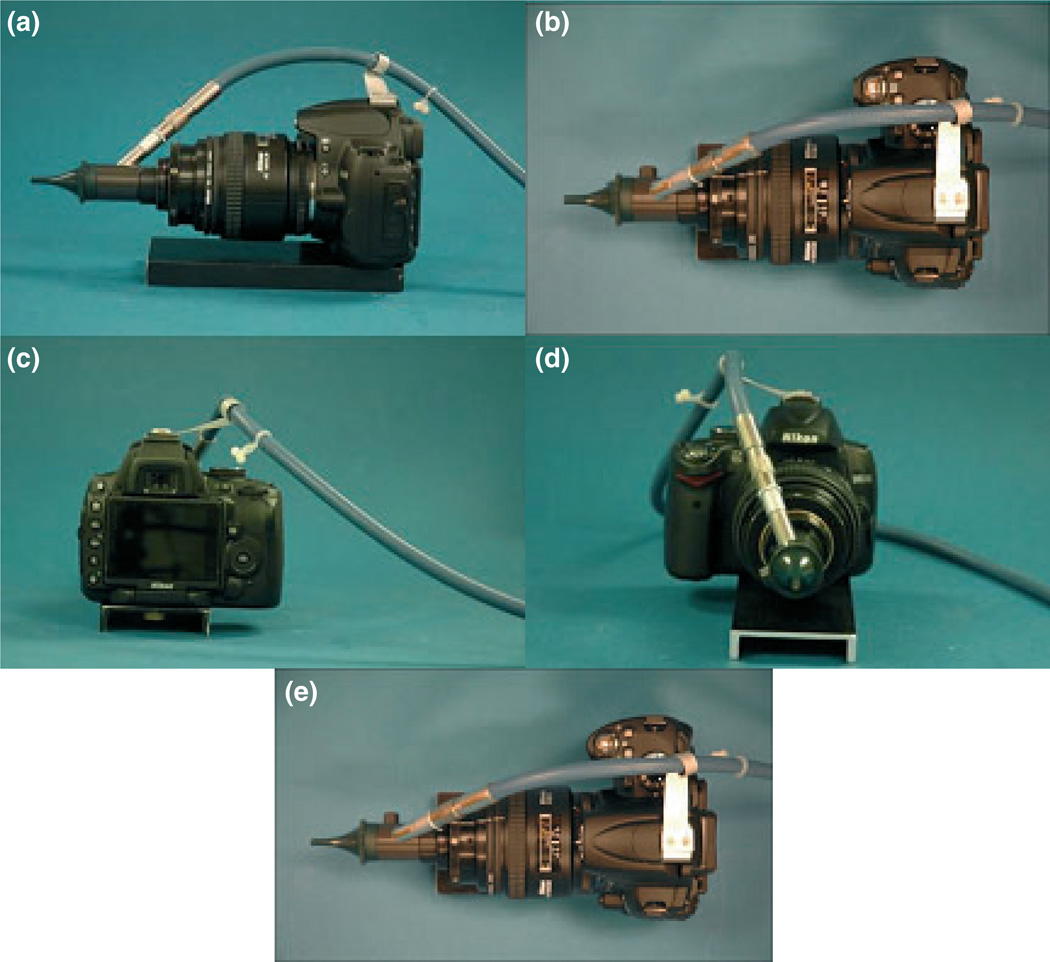
The filters from two neutral density filter adaptors were removed, leaving only the threaded camera lens interface. One was used to fixate both an aluminum plate and a lens to the camera and the other was used to fixate the lens focusing mount. The lens focusing mount allows for a 10 mm translation along its center axis. This was used to allow for minor focus adjustments needed for the varying ocular optics of the test subjects. The inner ring of the lens focusing mount, which is normally used to house a lens, was bored out to the largest outside diameter of the endoscope. This bore was then used to fix the otoscope to the focusing system. A C-clamp was machined from a Delrin rod to provide extra support within the mount. This system, once assembled, was then bonded together with ‘Lock-tite’ glue to avoid potential misalignment and improve rigidity. A small bracket was fabricated to hold the optical fiber up and away from the camera and the operator, creating a safer work environment. A platform to fixate the camera and endoscope was added to simplify operation so that the user needs only to manipulate the test subject to obtain views of the fundus (Fig. 3). The contact camera weighs approximately 1.3 kg.
Figure 3.
Images of the fundus imaging system. Left view (a), top view (b), back view (c), front view (d).
Funduscopy experiments
Fundus video and photography of two albino rats, two pigmented mice, two New Zealand white rabbits, and two albino guinea pigs were obtained. For all animals, pupils were dilated with one to two drops of Tropicamide Opthalmic Solution 1% (Bausch & Lomb, Tampa, FL) 30 minutes prior to imaging. Five minutes before imaging, anesthetic eye drops (two drops) were used in the eye to be tested (proparacaine hydrochloride 0.5%; Alcon Inc., Fort Worth, TX). A hypromellose ophthalmic demulcent solution (Gonak, Akorn Lake Forest, IL) was applied to the eye to provide a uniform optically transparent interface between the tip of the endoscope and the cornea of the subject. Two operators were present for the trials, one to operate the camera and the other to hold the animal. The camera was fixated to the work area and the animal was then moved until relevant fundus structures were seen in the field of view. Only the right eye was imaged in each animal for testing. Depending on the animal, light intensity was adjusted to optimize contrast of retinal structures. This was carried out by interfacing the camera to the eye and determining a visual threshold of saturation in the displayed images. Ten to fifteen images and sixty-seconds of video were taken of each animal.
RESULTS
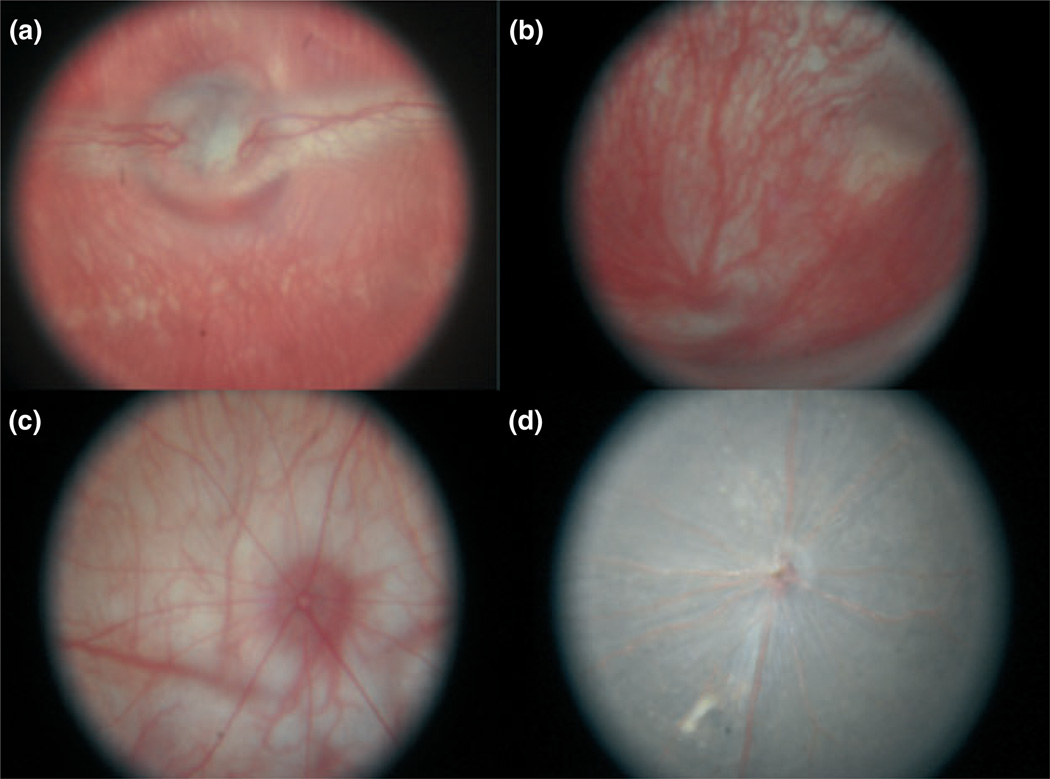
Typical digital images of the fundus of a mouse, rat, guinea pig, and rabbit are shown (Fig. 4). Fundus images were obtained from all animals in this study. Contrast of retinal vasculature and overall image quality varied from one animal to another, as the axial length, ocular optics, and retinal reflectance varied significantly across species, requiring adjustment of the light intensity and focus to optimize the image quality for each animal. As the image covered 72% of the field of the camera, the estimated definition of the fundus image is 8.7 Mega pixels. In all images, key posterior segment anatomical structures were clearly resolved, such as the optic nerve, retina, and retinal and choroidal vessels. In addition, the system was operated on different occasions by three engineers and two physicians, all without receiving any prior training, exhibiting a significant ease of use and negligible learning curve of the system. Total time for image acquisition was five minutes per animal.
Figure 4.
(a) Image of a normal 9-month-old albino rabbit (AL = 18 mm). The optic nerve, mylinated nerve fibers, and retinal vessels are present. Deeper choroidal vessels are also present. (b) Image of a normal 3-month-old guinea pig (AL = 9 mm). (c) Image of a normal 10-week-old rat (AL = 6 mm). (d) Image of a normal pigmented 8-week-old mouse (AL = 2.5 mm).
DISCUSSION
The contact endoscopic imaging system demonstrated is compact, relatively inexpensive, and easy to use, and was assembled almost entirely with off-the-shelf components. Variation between animals required adjustment of the light intensity to optimize the image quality, and even still, the outer 10% of the images suffered slightly from aberrations. The system provides high-resolution wide-field images of mouse, rat, guinea pig, and rabbit fundus, animals with a wide range of axial lengths. With modification, this system can likely be used in larger animals and potentially humans as well.
ACKNOWLEDGMENTS
The authors would like to thank Eleut Hernandez LAT for scientific support and Izuru Nose BSEE of the Ophthalmic Biophysics Center for technical assistance. Supported in part by NIH P30EY14801 (Center Grant); the Florida Lions Eye Bank; an unrestricted grant from Research to Prevent Blindness, Morris Morgenstern Foundation, Palm Beach, FL and the Henri and Flore Lesieur Foundation.
REFERENCES
- 1.Cohan BE, Pearch AC, Jokelainen PT, et al. Optic disc imaging in conscious rats and mice. Investigative Ophthalmology & Visual Science. 2003;44:160–163. doi: 10.1167/iovs.02-0105. [DOI] [PubMed] [Google Scholar]
- 2.DiLoreto D, Jr, Grover DA, del Cerro C, et al. A new procedure for fundus photography and fluorescein angiography in small laboratory animal eyes. Current Eye Research. 1994;13:157–161. doi: 10.3109/02713689409042411. [DOI] [PubMed] [Google Scholar]
- 3.Hawes NL, Smith RS, Chang B, et al. Mouse fundus photography and angiography: a catalogue of normal and mutant phenotypes. Molecular Vision. 1999;5:22–30. [PubMed] [Google Scholar]
- 4.Nakamura A, Yokoyama T, Kodera S, et al. Ocular fundus lesions in systemic lupus erythematosus model mice. Japanese Journal of Ophthalmology. 1988;42:345–351. doi: 10.1016/s0021-5155(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 5.Berendschot TTJM, DeLint PJ, Van Norren D. Fundus reflectance- historical and present ideas. Progress in Retinal and Eye Research. 2003;22:171–200. doi: 10.1016/s1350-9462(02)00060-5. [DOI] [PubMed] [Google Scholar]
- 6.Rudnicka AR, Burk ROW, Edgar DF, et al. Magnification characteristics of fundus imaging systems. Ophthalmology. 1998;105:2186–2192. doi: 10.1016/S0161-6420(98)91214-3. [DOI] [PubMed] [Google Scholar]
- 7.Ozerdem U. A simple nonmydriatic self-retinal imaging procedure using a Kowa Genesis-D hand-held digital fundus camera. Ophthalmic Research. 2009;42:125–127. doi: 10.1159/000226258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rol P, Jenny R, Beck D, et al. Optical properties of miniaturized endoscopes for ophthalmic use. Optical Engineering. 1995;34:2070–2077. [Google Scholar]
- 9.Paques M, Guyomard J-L, Simonutti M, et al. Panretinal, high-resolution color photography of the mouse fundus. Investigative Ophthalmology & Visual Science. 2007;48:2769–2774. doi: 10.1167/iovs.06-1099. [DOI] [PubMed] [Google Scholar]
- 10.Guyomard J-L, Rosolen S, Paques M, et al. A low-cost and simple imaging technique of the anterior and posterior segments: eye fundus, ciliary bodies, iridocorneal angle. Investigative Ophthalmology & Visual Science. 2008;49:5168–5174. doi: 10.1167/iovs.07-1340. [DOI] [PubMed] [Google Scholar]
- 11.Copland D, Wertheim M, Armitage W, et al. The clinical time-course of experimental autoimmune uveoretinitis using topical endoscopic fundal imaging with histologic and cellular infiltrate correlation. Investigative Ophthalmology & Visual Science. 2008;49:5458–5465. doi: 10.1167/iovs.08-2348. [DOI] [PubMed] [Google Scholar]
- 12.Gliss C, Parel J-M, Flynn JT, et al. Towards a miniaturized fundus camera. Journal of Biomedical Optics. 2004;9:126–131. doi: 10.1117/1.1631313. [DOI] [PubMed] [Google Scholar]
- 13.Yannuzzi LA, Ober MD, Slakter JS, et al. Ophthalmic fundus imaging: today and beyond. American Journal of Ophthalmology. 2004;137:511–524. doi: 10.1016/j.ajo.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 14.Fonda S, Melli M, Neroni M, et al. Recent developments in eye fundus imaging for clinical application: television fluoroangiography and new technologies. Graefe’s Archive for Clinical & Experimental Ophthalmology. 1983;220:66–70. doi: 10.1007/BF02133872. [DOI] [PubMed] [Google Scholar]