Abstract
Context:
Central pancreatectomy has gained popularity in the past decade as treatment of choice for low malignant potential tumor in the midpancreas due to its ability to achieve optimal preservation of pancreatic parenchyma. Simultaneously, advancement in minimally invasive approach has contributed to numerous novel surgical techniques with significantly lower morbidity and mortality. With the purpose of improving patient outcomes, we describe a laparoscopic assisted central pancreatectomy with pancreaticogastrostomy as an alternative method to the previously described open central pancreatectomy with roux-en-y pancreaticojejunostomy reconstruction.
Case Report:
A 39 year old man presented to our clinic with a 2.5 cm neuroendocrine tumor at the neck of the pancreas. Laparoscopic assisted central pancreatectomy with pancreaticogastrostomy reconstruction was successfully performed. Operative time was 210 minutes with blood loss of 200 ml. Postoperative course was uneventful except for a minimal pancreatic leak which was controlled by an intraoperatively placed closed suction drain. At 2 week follow up, patient was asymptomatic with well preserved pancreatic endo and exocrine functions. Permanent pathology findings showed a well differentiated neuroendocrine tumor with negative margins and nodes.
Conclusions:
Laparoscopic assisted central pancreatectomy with pancreaticogastrostomy reconstruction is feasible and safe for a centrally located tumor. Laparoscopic assisted technique facilitates application of minimally invasive approach by increasing surgical feasibility in typically complex pancreatic operations.
Keywords: Laparoscopic, central pancreatectomy, pancreaticogastrostomy
Introduction
Advantages of minimally invasive approach in general surgery have been widely recognized. Improved cosmesis, reduced postoperative pain and narcotics use, reduced immune system attenuation, rapid return of gastrointestinal function, shorter hospital stay, and lower incisional hernia rate are several factors favoring laparoscopy. While extensive use of minimally invasive technique in surgery for obesity, its role in pancreatic surgery is still controversial with only few centers in the world have reported their experience.
Open pancreatic surgery for endocrine tumors is known to be associated with high morbidity rate varying between 15 and 45 %[1,2]. Pancreatoenteral anastomosis has been considered the most critical step and represents the main cause of morbidity and mortality. Pancreaticogastrostomy (PG) and pancreaticojejunotomy (PJ) are two most popular techniques that are used to reestablish the gastrointestinal continuity following resection. Controversies regarding the better technique exist and review of the literature showed data that is almost exclusively originated from Whipple operation.
In an attempt to reduce morbidity and improve patient outcomes, we describe an alternative approach of laparoscopic assisted central pancreatectomy with retrogastric pancreaticogastrostomy reconstruction. Current opinion in the literature between those two pancreatic anastomosis techniques is discussed.
Case Report
A 39 year old man with no previous abdominal surgery was incidentally found to have a 2.5 cm mass in the neck of the pancreas on a computed tomography (CT) scan evaluation for acute diverticulitis. Past medical history was only significant for hypertension and gastroesophageal reflux disease. At the time of diagnosis, patient denied symptoms such as diarrhea, hypoglycemia, intractable peptic ulcer, weight loss, fever or migratory skin rash. CT guided fine needle aspiration biopsy confirmed a nonfunctional neuroendocrine tumor. Due to its malignant potential, decision was made to proceed with a surgical resection.
Patient was taken to the operating room and positioned flat with 60 degree left side up supported by a bean bag. After adequate administration of general anesthesia, pneumoperitoneum was established using Hasson's technique through a 10 mm left paramedian port. Two additional 5 mm working ports were placed on either side to create adequate triangulation toward the pancreas (Fig. 1). A 30 degree telescope was then inserted to inspect the peritoneal cavity. No macroscopic pathology was observed throughout the peritoneal surface and on the liver region. A handport device was then placed in the midline under direct vision.
Fig. 1.
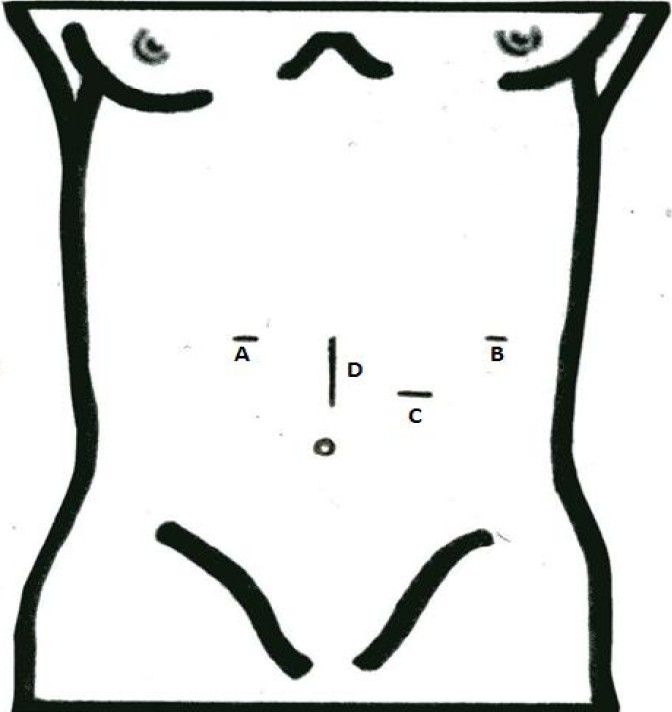
Laparoscopic trocars and handport placement. A, B = 5mm trocars for laparoscopic grasper and tissue dissector, C = 10mm trocar for telescope, and D = Handport.
Dissection was initiated with splenic flexure mobilization using a laparoscopic electrocautery. The lesser sac was carefully entered by elevating the stomach and dividing the gastrocolic ligament. The pancreas was then inspected and solitary lesion within the neck of the pancreas was clearly visualized (Fig. 2). Intraoperative ultrasound showed a 2.5 cm tumor located in the neck of the pancreas over the superior mesenteric vein (SMV)-splenic vein confluence. The lesion was found to be full thickness involving the major pancreatic duct rendering enucleation not feasible. After complete peripancreatic tissue dissection with careful attention to the superior mesenteric and splenic veins, central pancreas was transected using LigaSure™ sparing a large healthy appearing pancreatic tail. The proximal cut edge of the pancreas was inspected to ensure adequate hemostasis. The resected portion of the pancreas was placed in an impervious bag and retrieved through the handport.
Fig. 2.
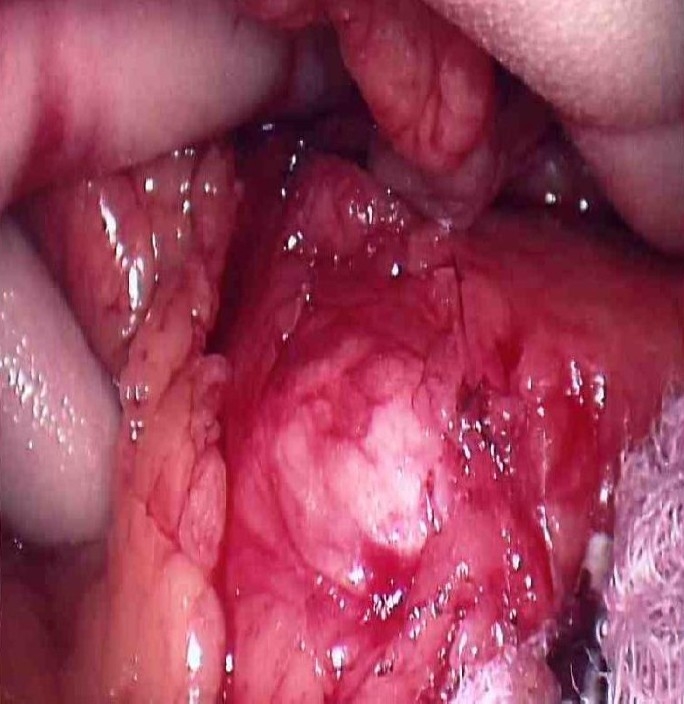
Solitary lesion within the neck of the pancreas, 2.5 mm in diameter. Intraoperative ultrasonography showed a full thickness lesion involving the major pancreatic duct rendering enucleation not feasible.
The remaining pancreatic tail was then elevated for a short distance to approximate the posterior wall of the stomach in preparation for the pancreaticogastrostomy anastomosis. Pancreatic duct was then cannulated using a 3 Fr Geenan™ stent (Fig. 3) and a retrogastric pancreaticogastrostomy was performed using interrupted silk sutures in a single layer fashion (Fig. 4). Two Blake drains were placed in the lesser sac. Specimen weighted 20 grams showing neuroendocrine tumor features with negative margins on frozen section. Proton pump inhibitor and octreotide were administered postoperatively.
Fig. 3.
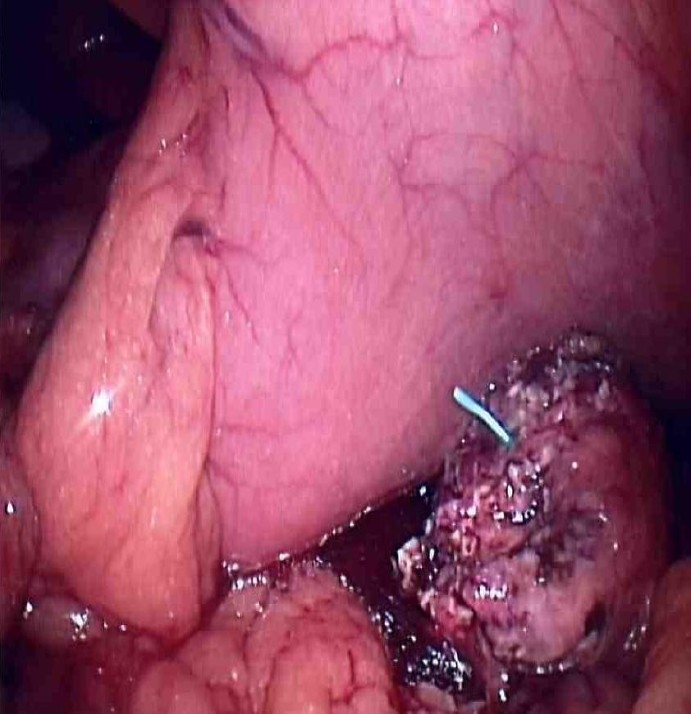
Cannulation of the distal pancreatic duct with a 3 Fr Geenan™ stent. The remaining distal pancreas was approximated to the posterior wall of stomach (has been decompressed with a nasogastric tube) in preparation for pancreaticogastrostomy reconstruction. Stent was utilized to help maintain the integrity of the newly created anastomosis.
Fig. 4.
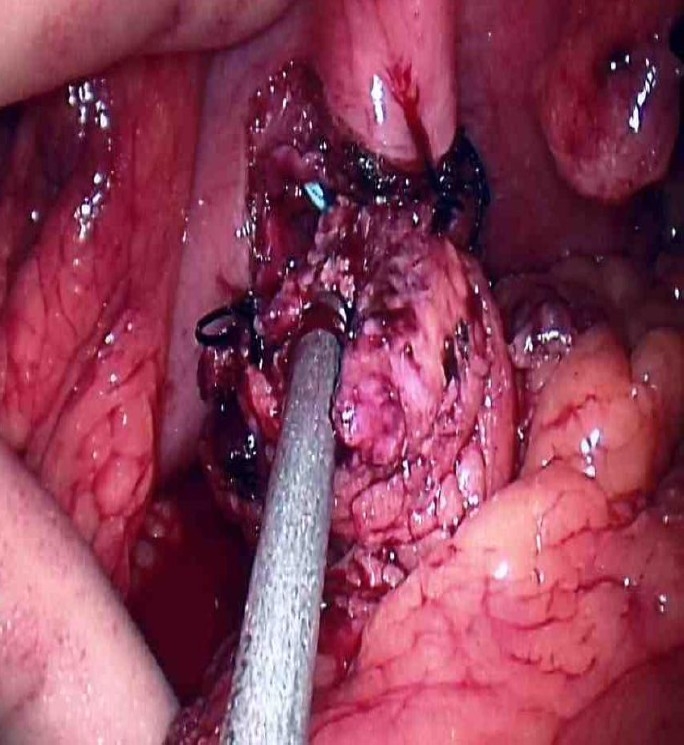
Retrogastric pancreaticogastrostomy was performed with interrupted silk sutures in a single layer fashion. The use of handport significantly improved feasibility to perform the anastomosis. Nasogastric tube was left in place for gastric decompression postoperatively.
Laparoscopic assisted central pancreatectomy was performed successfully with operative time of 180 minutes and blood loss of 200 ml. Postoperatively recovery was unremarkable and the patient was able to ambulate on postoperative day (POD) 1. Nasogastric tube was removed by the second POD. A slight increase in drain output up to 200 ml/24 hours was noticed following introduction of regular diet on POD 5. Drain fluid showed mildly elevated amylase and lipase. The patient clinical condition continued to improve without symptoms until hospital discharge on POD 9. Permanent pathology report showed a well differentiated pancreatic endocrine neoplasm of uncertain potential behavior with negative peripancreatic lymphnodes. Three days following discharge, patient presented to the emergency room due to drain malfunction which was simply managed by 2 cm drain retraction and reattachment. Repeat CT scan showed a minimal peripancreatic fluid collection around the surgical bed without oral contrast extravasation. Abdominal drains were removed in 2 weeks postoperatively and the patient continued to do well at 3 month follow-up without any signs of pancreatic insufficiency.
Discussion
Guillemin and Bessot first described the concept of dividing the pancreatic neck with pancreaticojejunal anastomosis in 1957 for treatment of chronic calcifying pancreatitis[3]. In 1959, Letton and Wilson desribed this modality for the treatment of traumatic rupture of the pancreatic neck[4]. Later, this concept became an attractive alternative technique for a benign or low-grade malignant tumor located in the middle portion of the pancreas.
For a neuroendocrine tumor of the pancreas, surgery is currently still considered to be the only potentially curative treatment. Conventional surgical treatment consists of enucleation, total pancreaticoduodenectomy or distal pancreactectomy with/without splenectomy. Low malignant potential tumor located in the neck or proximal pancreas such as in this case constitutes a surgical dilemma since enucleation is not always feasible whereas pancreaticoduodenectomy or extended distal pancreatectomy inevitably cause significant parenchymal loss and may result in impaired endocrine and exocrine functions. Incidence of new onset diabetes mellitus after Whipple procedure for periampullary tumors has been 10-15%, and up to 40% in patients with chronic pancreatitis[5–7]. For an extended distal pancreatectomy in the setting of chronic pancreatitis, the rate of new onset diabetes was reported as high as 90% since distal resection results in greater loss of islet cell (60-90%)[5–8]. In an effort to minimize the extent of healthy parenchymal loss, many authors have proposed a limited resection while maintaining oncologic principles of tumor excision[9–11]. Mustapha Adham et al reported absence of de novo diabetes mellitus following open central pancreatectomies in the largest series of 50 patients[12].
While a splenic preserving distal pancreatectomy is possible, the dissection is frequently tedious and becomes increasingly difficult when pancreatitis precedes the operation. By avoiding distal pancreatic dissection, central pancreatectomy offers additional benefit of splenic preservation with its immunologic advantage especially in patient with pre-existing impaired immune system.
Pancreatic reconstruction after a central pancreatectomy can be accomplished by either pancreaticojejunostomy or pancreaticogastrostomy. In our opinion, PG appears to be a more natural way to reestablish the foregut continuity due to the close anatomical relationship between the distal pancreas and posterior wall of the stomach allowing for a direct apposition and tension free anastomosis. More extensive blood soppy in the stomach wall compared to the jejunal wall also favors anastomotic integrity by reducing probability for tissue ischemia[13,14]. PG eliminates the need for small bowel resection to create a roux-en-y jejunal loop, therefore resulting in less risk for enteroenteric anastomotic complications.
Moritz N Wente et al explained that based on metanalysis of 3 randomized controlled trials, no significant difference was found between PG and PJ in term of postoperative complications, pancreatic fistula, intrabdominal collection or mortality[5]. The use of PG has been criticized because the inactivation of pancreatic amylase by the low gastric pH can theoretically contribute to exocrine insufficiency. However, evidence by Kim and colleagues showed no increase in clinically relevant exocrine insufficiency comparing PG to PJ after PD[4,15].
Despite all advantages of central pancreatectomy, in an expense of a less invasive tissue resection, a more careful examination of frozen sections to verify free resection margins is mandatory. Positive margin requires extended resection until convincing tumor free parenchyma is confirmed.
Conclusion
Regardless of more technically demanding laparoscopic pancreatic resection compared to open technique, laparoscopic central pancreatectomy is a safe and effective technique for low malignant potential tumor in the neck and proximal body of the pancreas while optimally preserving pancreatic function and the spleen. We advocate the use of pancreaticogastrostomy reconstruction over pancreaticojejunostomy due to anatomical advantage, technical simplicity, reduction in operative time. Hand assisted technique improves feasibility while maintaining the benefits of minimally invasive approach in typically complex pancreatic operations.
References
- 1.Owen NJ, Sohaid SA, Peppercorn PD, et al. MRI of neuroendocrine pancreatic tumors. Br J Radiol. 2001;74:968–973. doi: 10.1259/bjr.74.886.740968. [DOI] [PubMed] [Google Scholar]
- 2.Van NY, Vandaele S, Opde BB, et al. Neuroendocrine tumors of the pancreas: benefits of new technologies. Surg Endosc. 2003;17:1658–1662. doi: 10.1007/s00464-002-9268-x. [DOI] [PubMed] [Google Scholar]
- 3.Christein JD, Kim AW, Goldshan MA, et al. Central pancreatectomy for the resection of benign or low malignant potential neoplasm. World J Surg. 2003;27(5):595–598. doi: 10.1007/s00268-003-6848-4. [DOI] [PubMed] [Google Scholar]
- 4.Takada T, Yasuda H, Uchiyama K, et al. Pancreatic enzyme activity after pylorus-preserving pancreaticoduodenectomy reconstructed with pancreaticogastrostomy. Pancreas. 1995;11:276–282. doi: 10.1097/00006676-199510000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Frey CF, Child CG, Frey W. Pancreatectomy for chronic pancreatitis. Ann Surg. 1976;1984:403–414. doi: 10.1097/00000658-197610000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jalleh RP, Williamson RCN. Pancreatic exocrine and endocrine function after operations for chronic pancreatitis. Ann Surg. 1992;216:656–662. doi: 10.1097/00000658-199212000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slezak LA, Andersen DK. Pancreatic resection: Effects on glucose metabolism. World J Surg. 2001;25:452–460. doi: 10.1007/s002680020337. [DOI] [PubMed] [Google Scholar]
- 8.Hutchins RR, Hart RS, Pacifico M. Long term result of distal pancreatectomy for chronic pancreatitis in 90 patients. Ann Surg. 2002;236:612–618. doi: 10.1097/00000658-200211000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperti C, Pasquali C, Ferronato A, et al. Median pancreatectomy for tumors of the neck and body of the pancreas. J Am Coll Surg. 2000;190:715–716. doi: 10.1016/s1072-7515(00)00286-6. [DOI] [PubMed] [Google Scholar]
- 10.Paretensky C, Apa D, Marchal F. Median pancreatectomy with pancreaticogastric anastomosis in pancreatic neoplasm. Chirurgie. 1998;123:363–367. doi: 10.1016/s0001-4001(98)80006-8. [DOI] [PubMed] [Google Scholar]
- 11.Efron DT, Lillemore KD, Cameron JL, et al. Central pancreatectomy with pancreatogastrostomy for benign pancreatic pathology. J Gastrointest Surg. 2004;8:532–538. doi: 10.1016/j.gassur.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Adham M, Giunippero A, Hervieu V, et al. Central pancreatectomy-single center experience of 50 cases 2008; 143 (No. Arch Surg. 2008;143(2):175–180. doi: 10.1001/archsurg.2007.52. [DOI] [PubMed] [Google Scholar]
- 13.Sikora SS, Posner MC. Management of the pancreatic stump following pancreaticoduodenectomy. Br J Surg. 1995;82:1590–1597. doi: 10.1002/bjs.1800821205. [DOI] [PubMed] [Google Scholar]
- 14.Poon RTP, Lo SH, Fong D, et al. Prevention of pancreatic anastomostic leakage after pancreaticoduodenectomy. Am J Surg. 2002;183:42–52. doi: 10.1016/s0002-9610(01)00829-7. [DOI] [PubMed] [Google Scholar]
- 15.Kim SW, Youk EG, Park YH. Comparison of pancreatogastostomy and pancreatojejunostomy after pancreatoduodenostomy performed by one surgeon. World J Surg. 1997;21(6):640–643. doi: 10.1007/s002689900286. [DOI] [PubMed] [Google Scholar]


