Figure 1. Distinct functions of Hsp90 during viral replication.
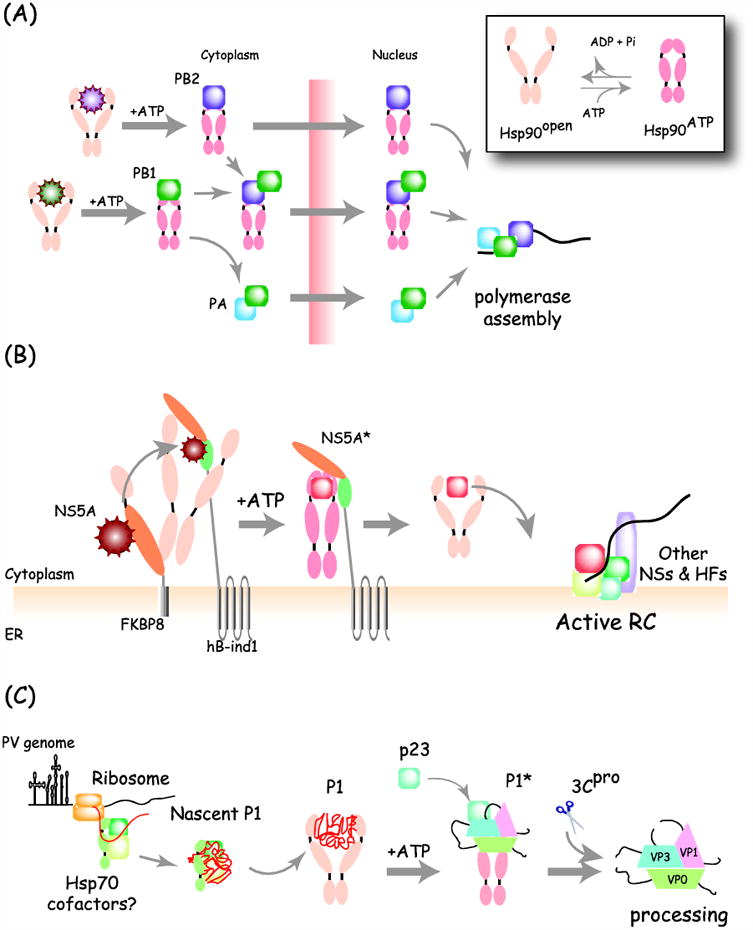
(A) Hsp90-facilitates assembly of Influenza polymerase. Newly made influenza PB2 interacts with Hsp90 in the cytoplasm and the complex translocates into the nucleus. Another polymerase subunit PB1, which itself interacts with Hsp90, is also transported into the nucleus together with the PB2/Hsp90 complex. Once in the nucleus, Hsp90 dissociates as PB2 binds to PB1/PA to form the trimeric active polymerase. Inset: the two conformations of Hsp90 are regulated by its ATPase cycle. (B) Formation of a multi-chaperone complex with non-structural protein NS5A during HCV replication. HCV NS5A assembles into a complex containing Hsp90, FKBP8 and hB-ind1. Interaction with FKBP8 is required for NS5A entry into the Hsp90 cycle whereas hB-ind1 action stimulates dissociation from Hsp90 and correct NS5A folding (NS5A*), leading incorporation into an active replication complex (RC) containing other NS proteins and host factors (HFs). (C) Role of Hsp90 in picornavirus capsid maturation. Picornavirus capsid precursor poly-protein P1 binds associates with Hsp90, likely following an upstream interaction with Hsp70. Hsp90 is required to fold P1 to a mature conformation (P1*) that is competent for proteolytic cleavage by the viral protease (3CPro) into the mature capsid protein. The processed capsid proteins no longer interact with Hsp90.
