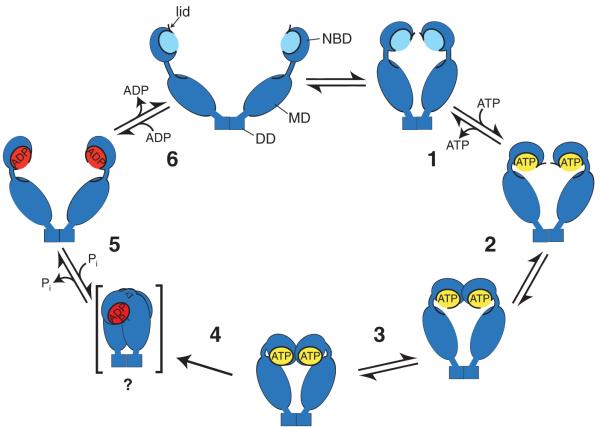
Figure 1 The ATPase cycle of Hsp90 proteins. Model of the ATPase cycle of Hsp90 proteins as revealed by kinetic analysis of ATP binding and/or conformational changes of yeast cytosolic Hsp82, human endoplasmic reticulum GRP94, human mitochondrial TRAP1 and E. coli HtpG. Hsp90 proteins proceed through two distinct intermediates before reaching the γ-phosphate cleavage–competent conformation. Forward and backward reaction rates are different for the individual Hsp90 proteins analyzed and may be subjected to regulation by cochaperones such as Aha1. A proposed compact post-hydrolysis state visible in EM seems to be transient and is not detectable by bulk measurements like SAXS or HX-MS.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.