Abstract
Objective:
Assessment of the analgesic effect of an agent in an experimental pain model permits a level of control not possible in a clinical pain setting and is an ideal approach for evaluation of analgesic drugs. The aim of the present study was to establish a simple and reliable method of producing experimental pain, which can be used for screening of various analgesic agents.
Materials and Methods:
The standardized method was followed in all cases, by recording thermal pain threshold in seconds in 24 healthy volunteers using hot air source at two different speeds, which is equipped in an acrylic-made chamber adjustable to three different levels. Reproducibility of the test procedure was evaluated by recording the thermal threshold parameter by a single observer on two sessions (interday reproducibility) and second observer on one session (interobserver reproducibility) separately. Validity of model was further tested by evaluating the analgesic effect of tramadol on 12 healthy volunteers.
Results:
Thermal pain model was found to produce low variability with coefficient of variation (CV) less than 10%. Interobserver and interday reproducibility were very good, as shown by Bland–Altman plot, with most of the values within ± 2SD. There was a significant increase in pain threshold time with use of tramadol as compared to placebo which was statistically significant (P < 0.05).
Conclusion:
The newly developed pain model offers a stable and sensitive method for the early assessment of analgesic activity.
Keywords: Analgesia, experimental, heat pain, pharmacodynamics, tramadol
Introduction
Pain is the most prevalent health care problem, and characterization of pain is of major importance in the diagnosis and choice of treatment.[1] Studies of analgesic efficacy in patients already suffering pain raise ethical issues. In clinical practice, the symptoms of the underlying disease and complaints relating to psychological, cognitive, and social aspects of the illness, as well as systemic reactions such as fever and general malaise confound the characterization of pain.[2] Experimentally induced pain avoids some ethical issues. Experimentally induced pain, with its greater precision and the ability to use healthy subjects in a controlled environment, may be a better model in preclinical investigation of analgesics. There is, however, considerable debate over the degree to which pain in healthy subjects can be considered analogous to clinical pain and no consensus has been reached as to the optimal experimental pain model.
Several experimental approaches have been used in the early screening of new analgesics. Commonly, these tests measure subjective pain after pain stimuli. However, these approaches are limited mostly because of the poor standardization of subjective pain ratings.[3]
An alternative for determining the effect of analgesics is quantitative sensory testing (QST). QST has a particular advantage of being a functional test that provides a quantitative pain stimulus and assesses the subject's individual response to the stimulus.[4,5] The repeatability of the visual analog scale has been shown to be poor in a setting of human experimental heat pain compared with thermal QST.[6]
The aim of the present study was to establish a simple and reliable method of producing experimental pain using QST as a functional test, which could be used to distinguish the analgesic effects of drugs from placebo effects.
Materials and Methods
Twenty four healthy participants (6 women and 18 men) aged 24–33 years, with a body mass index (BMI) 19.5–25.9 kg/m2 were studied. Exclusion criteria were pre-existing neurological disease, any acute or chronic drug or alcohol use, diabetes mellitus, known allergy to tested substances, inability to communicate in the local language, inability to perform the test as per protocol procedure, and prior wounds or fractures on the tested extremity. The volunteers were given a short explanation of the purpose of the research and a description of the procedure to be followed. They were further given a description of any reasonably forseeable risks and discomforts. Written consent to participate was obtained from each volunteer. Before it was initiated, the study was approved by the Institutional Ethics Committee on Research Involving Human Beings.
A hot air analgesiometer was used to deliver the thermal pain stimulus for the study. The hot air analgesiometer was designed and developed by the Department of Clinical Pharmacology and Therapeutics, Nizam's Institute of Medical Sciences, Hyderabad (India). It delivers variable, quantifiable, and reproducible heat stimulus to induce thermal pain on the volar surface of subjects’ forearm. The apparatus is made up of two acrylic chambers. The lower chamber (hand resting chamber - A), rectangular in shape of length (37.4 cm) × width (25.3 cm) × height (21 cm), with an opening on both sides to place the hand. The upper chamber (hot air delivery chamber - B), having dimension of – length (12 cm) × width (12 cm) × height (21 cm) is placed on the centre of the lower chamber. The upper chamber is fitted with an additional freely movable acrylic chamber (C) to raise the height of the hot air source to a maximum height of 50.5 cm [Figure 1]. The height of the upper chamber (B) can be adjusted with an adjustment knob at three different levels by raising the movable chamber (C). This chamber can be moved to 3 levels, namely Level 1 (short) = 36.5 cm, Level 2 (medium) = 43.5 cm, and Level 3 (high) = 50.5 cm. When the movable chamber is placed at short level, the distance between the heat source and the site (volar surface of forearm) of heat application is shortest, while the distance between the heat application site from heat source is maximum when chamber is raised to high level. There is a 7.8 cm diameter circular aperture at the top of the hot air delivery chamber (C), to insert the nozzle of the hair drier (source of hot air with 1200 watts power). The blow of air from the hair drier can be adjusted to two speeds – low and high. On both side walls of lower chamber A, there are three apertures, each 3 cm diameter, to vent the excess heat generated during the test procedure.
Figure 1.
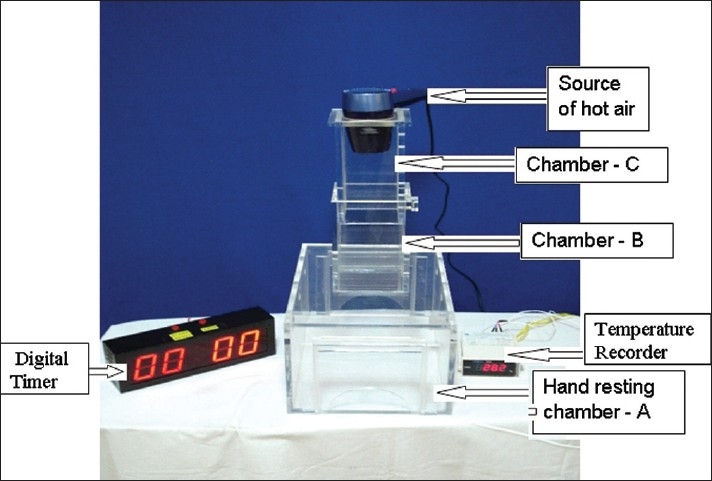
Hot-air analgesiometer
To record the temperature change (in °C), a highly sensitive temperature recording sensor [Nippon Instruments (India) Pvt. Ltd] was placed in a slot provided in at the height of 10 cm from the base of the lower chamber A. An electronic digital clock was used to record the reaction time in seconds.
The entire test procedure was explained to the subjects a day before. After an overnight good sleep, the subjects were asked to relax and sit comfortably in a quite experimental room for about half hour before the test procedure. The subjects were asked to keep their nondominant hand exposing the volar aspect of the forearm into the lower chamber – A [Figure 2]. Initially, hot air was blown by adjusting the height of chamber B to the short level of 36.5 cm and blowing air at high speed. Without prior notice, the hair drier was turned on by an experimenter. The subjects were instructed to indicate as soon as they perceived the heat sensation as painful (pain threshold), immediately the hair drier was turned off and the experimental hand was removed from the chamber. The time elapsing between the turning on of the heat source and indication of heat sensation as painful was measured in seconds by a digital clock. Depending on the response of the subject to hot air stimuli, the height of the chamber B and magnitude of hot air blow were modified, to find out the optimum test condition for each subject. The test procedure was repeated at least three times with inter-stimulus interval of 5 min. The mean of the three measurements was determined for analysis.
Figure 2.
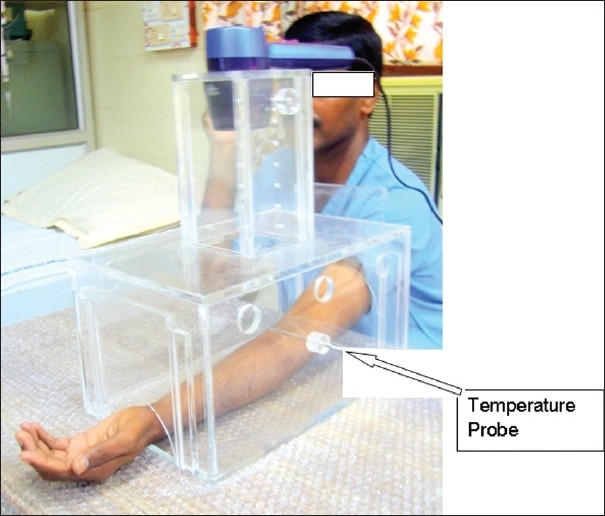
Delivery of hot-air pain stimulus to study participant
It was observed that data obtained on pain threshold time in seconds were reproducible and consistent with high speed and at short level. Reproducibility of the method with high speed and at short level, across sessions and subjects, was evaluated by recording the thermal threshold parameter by a single observer on two sessions (interday reproducibility) and second observer in a separate single session (interobserver reproducibility) with high speed and at short level.
Twelve healthy male subjects (different from the 24 subjects studied during standardization) aged 22–35 years, with a BMI 21.0–25.4 kg/m2 were studied. The study was designed as randomized, double-blind, and placebocontrolled study in a cross-over design. The participants received either one capsule of 50 mg tramadol or similarly looking placebo in the morning after light breakfast. In between the administration of tramadol and placebo, 1 week washout was allowed. On the day of experiment, procedure as described earlier to elicit pain was carried out. As the results were more consistent with high speed and at short level, these conditions were used throughout the experimentation. Pain threshold time in seconds was recorded at baseline (0 min) and then at 30, 60, 120, and 180 min after administration of drug.
During the application of the study drugs, a sedation score (0 = awake, 1 = tired, 2 = asleep but arousable, and 3 = non-arousable) was assessed every 10 min. All side effects were noted. To find out the role of anxiety in modulation of pain, we have recorded State/Trait Anxiety Inventory (STAI) anxiety scores in our study participants. STAI is a 40-item questionnaire which provides separate measure of state and trait anxiety with 20 questions each. The trait reflects the general tendency for experiencing anxiety, while state anxiety is a measure of the intensity of anxiety experienced at the time of assessment. Score range 20–80, with higher scores indicating higher levels of anxiety.
The data on thermal pain threshold were recorded in seconds and presented as mean (±SD) and coefficient of variation (CV). Bland–Altman plotting was performed for the assessment of method reproducibility.[7] The relative (positive or negative) differences between each pair of measurements were plotted against the mean of the pair to make sure that no obvious relation appeared between the estimated values of mean and difference. The Bland–Altman analysis was done to compare the values of pain threshold time obtained by two observers separately. Similarly, the comparisons were also made to confirm the reproducibility by analyzing the pain threshold time values obtained on two sessions. The paired student t-test was used to compare the difference within the group and between the two groups; value of P < 0.05 was considered to indicate statistical significance. All the statistical analysis were performed using the Graph Pad PRISM software 4 (Graph Pad Software Inc., San Diego, CA, USA).
Results
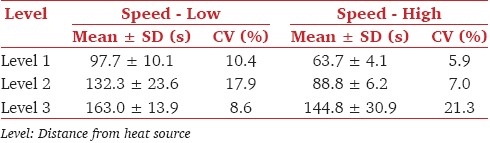
Results of the method standardized refer to a group of 24 healthy volunteers, aged 24–33 years, who were apparently healthy of the basis of their medical examination and laboratory investigations. In no case burn injury were observed. The pain threshold time obtained at three distance levels namely, level 1, level 2, and level 3 with two speeds – low and high is shown as Table 1. Except at level 3, with low-speed high variability in perceiving the reaction time was found when compared with high speed. When the pain threshold time was compared between highest distance level and shortest distance level of heat source at high speed/low speed, it was noticed that pain threshold time was higher with longer distance. At high speed, high variability in perceiving pain threshold time was found when the participants were exposed to medium and high level as compared to short level.
Table 1.
Mean pain threshold time and coefficient of variation (CV) for the experimental pain stimuli
The reproducibility of the method across sessions and subjects at short level and high speed, the data on interday and interobserver reproducibility on pain threshold time were studied. Relationship and Bland–Altman plot comparing the measurements from two sessions and two observers are shown [Figures 3 and 4]. In the Bland–Altman plot of inter-day and inter-observer measurement of pain threshold time, there was no significant difference in the values for reproducibility reported between the sessions and observers and the range of most of the values was within mean (± 2SD).
Figure 3.
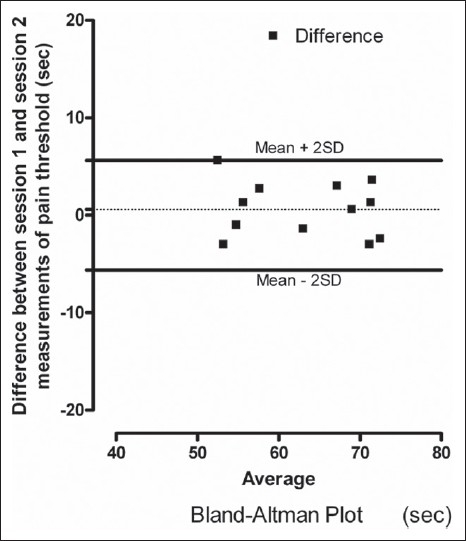
Bland–Altman plot showing session 1 and session 2 differences in measurements of heat pain thresholds
Figure 4.
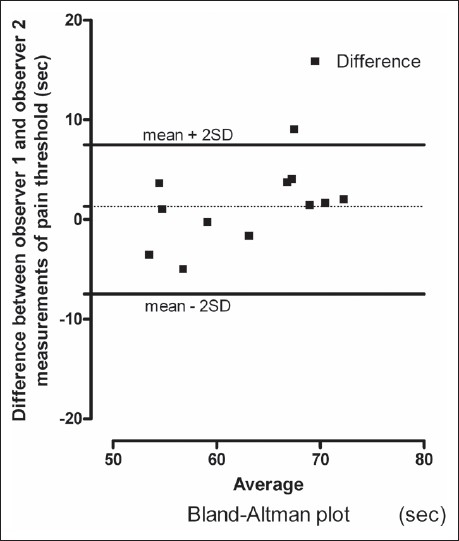
Bland–Altman plot showing observer 1 and observer 2 differences in measurements of heat pain thresholds
To confirm the validity of the method, we have used tramadol as a reference opioid analgesic. Twelve healthy male volunteers completed the study. Their demographic characteristics of the subjects were (mean ± SD): age 28.4 ± 6.5 years; height 169.6 ± 4.6 cm; weight 67.3 ± 6.8 kg; and body mass index, 23.2 ± 2.2 kg/m2. There was no significant difference in thermal pain threshold at baseline between placebo and tramadol (P > 0.05). Mean pain threshold values at each time point for placebo and tramadol are listed in Table 2. Figure 5 displays mean (±SEM) percent change from baseline pain threshold at each time point for placebo and tramadol.
Table 2.
Mean pain threshold time for placebo and tramadol across all time points
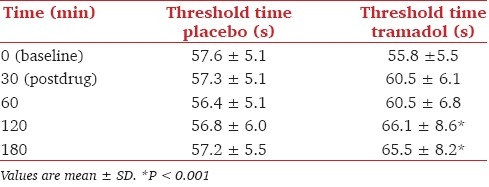
Figure 5.
Showing mean ± SEM percent change from baseline pain threshold time for placebo and tramadol
The analysis comparing placebo and tramadol revealed a significant increase in pain threshold time at 30, 60, 120, and 180 min, with a significant main effect of treatment at 120 and 180 min (P < 0.001). Figure 6 displays the mean of the difference in area under curve (AUC) between placebo and tramadol; significance was seen at 120 and 180 min (P < 0.01) only. Mild sedation was seen in some volunteers, but never exceeded sedation score 1.
Figure 6.
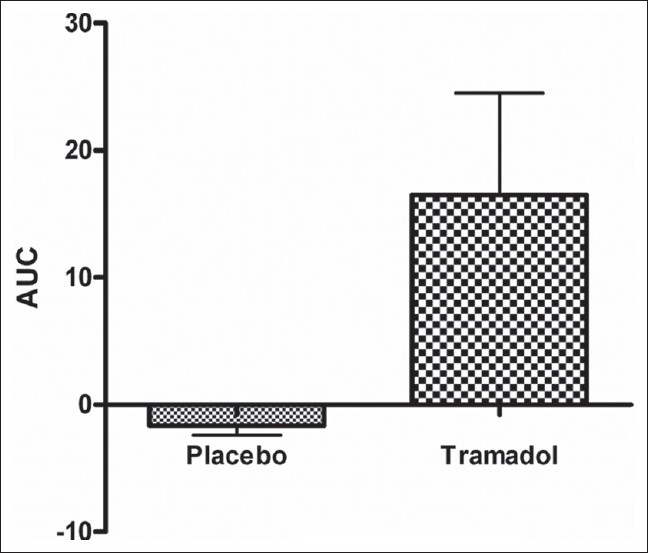
Displays the mean ± SEM of the difference in area under curve (AUC) between percent change from baseline pain threshold time for placebo and tramadol
The mean (±SD) STAI state and trait anxiety scores were 36.5 ± 1.3 and 37.1 ± 1.1, respectively. Anxiety scores did not have any effect on pain ratings.
Discussion
The present study describes an experimental pain technique in humans, which is sensitive to the tramadol at doses known to be effective in acute pain. The procedure is harmless, although a flare response was observed in vast majority of the subjects, no burn injuries occurred in any of our subjects. Moreover, it is simple to perform, cost effective, and requires few personnel.
In the present experimental model, the intensity of heat can be controlled by adjusting the knob of heat source at low or high and one can also adjust the distance between the heat source and stimulus site, allowing optimum and well-controlled stimulus delivery to the study participant. When the right model is chosen, one should bear in mind that the stimulation intensity in the experimental model should be well controlled, so that the impact on the pain system is stable.[2,8] This also ensures repeatability, which is crucial for good sensitivity toward analgesic modulation of pain in the model.[9]
One well-known index of accuracy of a method is the CV and CV of less than 10% are considered to be the hallmark of a good assay for a subjective phenomenon.[10] In the present study, we have reported CV less than 10% for levels 1 and 2 at high speed. Further, the data obtained for heat pain threshold on the relationship and Bland–Altman plot comparing inter-day and inter-observer measurements were to evaluate the reproducibility of experimental pain model across sessions and subjects. There was no significant difference in the values for reproducibility reported between the observers and between the sessions and most of the values range within mean (± 2SD) of the Bland–Altman plot. Reproducibility is an important factor in the testing of analgesics, where it is necessary to repeat the pain stimulation several times during active and placebo treatments. Earlier studies aimed at presenting data of heat-induced pain, assessed and reported intersession repeatability employing methods based on standard recognized statistical technique.[7]
Response to pain stimulus is highly subjective and varies from subject to subject, necessitating proper sample size estimation for evaluation of an analgesic drug on human participants. For method optimization and standardization, we have included total 24 healthy human volunteers in our experiment. Trials involving experimental pain often use small sample sizes because the variation of the outcome measures is less than in traditional clinical trials. Trials with fewer than 10–12 subjects are hard to test statistically and findings therefore questionable. However, it has been shown that experimental models with a high reproducibility, a sample size < 10, are powered to show the effect of analgesics.[9]
The major criticism of experimental pain techniques is short duration of exposure to the stimuli, which differs from clinical pain. The ability of this method to discriminate tramadol from placebo was attributed to the “tonic” nature of the stimulus. However, it is probably irrelevant whether the experimental pain stimulus is delivered as “phasic” or “tonic.” It is more important that the stimulus reaches sufficient intensity to produce pain sensation (burning quality due to activity in the unmyelinated C nociceptors) because it is the latter sensation that is reliably attenuated by both non-narcotic and narcotic analgesics.[11]
The tonic heat model also offers an important theoretical advantage compared to repetitive-phasic stimulation models. In the latter, the subject goes through an alternation of pain anticipation and pain relief states. There is ample evidence from human brain mapping studies that both anticipation and termination of a painful event can activate the brain reward system,[12,13] and that this process is modulated by dopaminergic mechanisms.[14] This interrelationship makes it difficult to disentangle pain-related and reward-related changes in dopamine receptor availability.
In the present study, we utilized the crossover design to compare the analgesic effect of tramadol and placebo in healthy subjects and single investigator performed all pain assessments. In general, parallel studies give a weaker statistical power than a cross-over design, demanding larger sample sizes.[15] In case of cross-over designed studies, it is important that the investigator is the same in all pain assessments, since gender and appearance of the investigator can influence the pain rating of the volunteers.[16]
The sensitivity of a given experimental model for detecting analgesia is affected by the method used to measure this pain. Hence, good sensitivity of a model is obtained by using a pain assessment that is reliable producing data with modest variance (noise). In the study by Thurauf et al., the value of objective pain assessment was shown since an effect of tramadol was found only on evoked brain potentials and not on pain ratings.[17] It is however important to note that although evoked potentials can be sensitive measure of nociceptive processes, they only measure a single dimension of pain. Pain is a multidimensional sensation and this is reflected better in the subjective pain measure. This limits the translation of analgesic effect on evoked brain potentials into effect on clinical pain measures. In the present study, we have used QST as a functional test which provides a reliable assessment of changes in pain thresholds. Also, thermal QST (heat and cold) allows a distinction between predominantly C-fiber activity and A-delta fiber activity.
The study duration of the drug was based on a consideration of t-max. Thus, tramadol, which has a t max of ~ 3 h, was tested for 3 h. There is strong evidence that for most analgesics, clinical analgesia is not a direct function of drug concentration. Therefore, the time-course of analgesic effect for analgesic drugs is characteristic of pharmacologic effect (analgesia), consistent with a role of an endogenous substance in the analgesic effect. In the present study, the time to peak effect after tramadol occurred between 2 and 3 h.
The kinetic profile is necessary to determine when it is optimal to perform the pain tests, bearing in mind that bad timing of the pain testing can jeopardize an otherwise well-designed trial. For opioids, it is particularly important to remember that they often need to cross the blood–brain barrier and enter the CNS to have analgesic effect. This causes a lag-time to the onset of analgesia. The study design should consider these different lag-times for different opioids. In the present study, considering the t-max of the tramadol, we have performed all pain assessments at 30, 60, 120, and 180 m after administration of the drug.
All subjects were given light breakfast since oral administration of tramadol with food does not significantly affect its rate or extent of absorption. We believe that fasting the subjects to increase the absorption of analgesics could introduce additional stress. Dietary manipulation can alter human pain sensitivity in that rapid increases in circulating glucose produce a decrease in the ability to tolerate pain.
We did not find a correlation between pain sensitivity and anxiety in this study. This seems to be in contrast with previous studies indicating a role of anxiety in the modulation of the pain experience.[18–21] The STAI anxiety scores in our participants ranged from low to moderate. We therefore conclude that in cases of low to moderate anxious subjects, state and trait anxiety do not have effect on the pain reports.
When opioids are applied in experimental pain, sedation is particularly troublesome since it can affect the pain scoring. In our study, mild sedation was seen in some volunteers, but never exceeded sedation score 1.
Conclusions
The present experimental pain model utilizing tonic heat stimulation of the volar aspect of the forearm, to mimic clinical pain, can discriminate between tramadol and placebo. This model is simple and requires inexpensive equipment. The model may therefore potentially allow analgesic effects of new compounds to be quantified in healthy volunteers, before proceeding to expensive clinical trials in acute and chronic pain sufferers.
Acknowledgments
The study was funded through the Indian Council of Medical Research (ICMR) fund, Government of India. We thank the Director, Nizam's Institute of Medical Sciences, for providing necessary infrastructure.
Footnotes
Source of Support: ICMR
Conflict of Interest: None declared.
References
- 1.Konig HH, Bernert S, Angermeyer MC. Health Status of the German Population: Results of a Representative Survey Using the euroqol questionnaire. Gesundheitswesen. 2005;67:173–82. doi: 10.1055/s-2005-857991. [DOI] [PubMed] [Google Scholar]
- 2.Drewes AM, Gregersen H, Arendt-Nielsen L. Experimental pain in gastroenterology: A reappraisal of human studies. Scand J Gastroenterol. 2003;38:1115–30. doi: 10.1080/00365520310004399. [DOI] [PubMed] [Google Scholar]
- 3.Posner J, Telekes A, Crowley D, Phillipson R, Peck AW. Effects of an opiate on cold-induced pain and the CNS in healthy volunteers. Pain. 1985;23:73–82. doi: 10.1016/0304-3959(85)90232-5. [DOI] [PubMed] [Google Scholar]
- 4.Brennum J, Dahl JB, Moiniche S, Arendt-Nielsen L. Quantitative sensory examination of epidural anaesthesia and analgesia in man: Effects of pre- and post-traumatic morphine on hyperalgesia. Pain. 1994;59:261–71. doi: 10.1016/0304-3959(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen JL, Kehlet H. Secondary hyperalgesia to heat stimuli after burn injury in man. Pain. 1998;76:377–84. doi: 10.1016/S0304-3959(98)00070-0. [DOI] [PubMed] [Google Scholar]
- 6.Yarnitsky D, Sprecher E, Zaslansky R, Hemli JA. Multiple session experimental pain measurement. Pain. 1996;67:327–33. doi: 10.1016/0304-3959(96)03110-7. [DOI] [PubMed] [Google Scholar]
- 7.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 8.Staahl C, Drewes AM. Experimental human pain models: A review of standardized methods for preclinical testing of analgesics. Basic Clin Pharmacol Toxicol. 2004;95:97–111. doi: 10.1111/j.1742-7843.2004.950301.x. [DOI] [PubMed] [Google Scholar]
- 9.Staahl C, Reddy H, Andersen SD, Arendt-Nielsen L, Drewes AM. Multi-model and tissue-differentiated experimental pain assessment: Reproducibility of a new concept for assessment of analgesics. Basic Clin Pharmacol Toxicol. 2006;98:201–11. doi: 10.1111/j.1742-7843.2006.pto_211.x. [DOI] [PubMed] [Google Scholar]
- 10.Youden WJ, Steiner EH, editors. Washington DC: Benjamin Franklin Station; 1975. Statistical manual of the official Association of Analytical Chemists. PO Box 540. [Google Scholar]
- 11.Stacher G, Steinringer H, Schneider S, Mittelbach G, Winklehner S, Gaupmann G. Experimental pain induced by electrical and thermal stimulation of the skin in healthy man: Sensitivity to 75 and 150 mg diclofenac sodium in comparison with 60 mg codeine and placebo. Br J Clin Pharmac. 1986;21:35–43. doi: 10.1111/j.1365-2125.1986.tb02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–7. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- 13.Seymour B, O’Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, et al. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat Neurosci. 2005;8:1234–40. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- 14.Menon M, Jensen J, Vitcu I, Graff-Guerrero A, Crawley A, Smith MA, et al. Temporal difference modeling of the blood-oxygen level dependent response during aversive conditioning in humans: Effects of dopaminergic modulation. Biol Psychiatry. 2007;62:765–72. doi: 10.1016/j.biopsych.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Garcia R, Benet M, Arnau C, Cobo E. Efficiency of the cross-over design: An empirical estimation. Stat Med. 2004;23:3773–80. doi: 10.1002/sim.2072. [DOI] [PubMed] [Google Scholar]
- 16.Gijsbers K, Nicholson F. Experimental pain thresholds influenced by sex of experimenter. Percept Mot Skills. 2005;101:803–7. doi: 10.2466/pms.101.3.803-807. [DOI] [PubMed] [Google Scholar]
- 17.Thurauf N, Fleischer WK, Liefhold J, Schmid O, Kobal G. Dose dependent time course of the analgesic effect of a sustained-release preparation of tramadol on experimental phasic and tonic pain. Br J Clin Pharmacol. 1996;41:115–23. doi: 10.1111/j.1365-2125.1996.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 18.Absi M, Rokke PD. Can anxiety help us tolerate pain? Pain. 1991;46:43–51. doi: 10.1016/0304-3959(91)90032-S. [DOI] [PubMed] [Google Scholar]
- 19.James JE, Hardardottir D. Influence of focus and trait anxiety on tolerance of acute pain. Br J Health Psychol. 2002;7:149–62. doi: 10.1348/135910702169411. [DOI] [PubMed] [Google Scholar]
- 20.Rhudy JL, Meagher MW. Fear and anxiety: Divergent effects on human pain thresholds. Pain. 2000;84:65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- 21.Tang J, Gibson SJ. A psychophysical evaluation of the relationship between trait anxiety, pain perception, and induced state anxiety. J Pain. 2005;6:612–9. doi: 10.1016/j.jpain.2005.03.009. [DOI] [PubMed] [Google Scholar]


