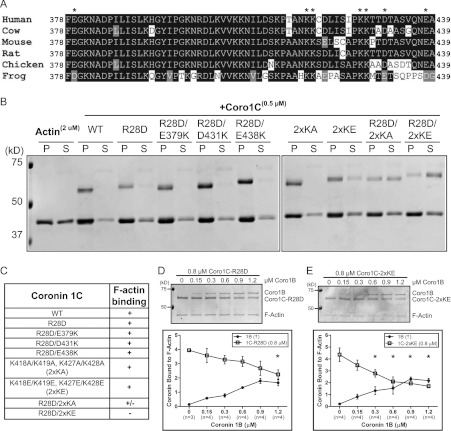
Figure 2. Coro1C harbours a second actin-binding site.
(A) Protein sequence alignment of the vertebrate Coro1C unique region. *Charged residues mutated for assays in (B). (B) Actin cosedimentation was performed using 2 μM actin alone or with 0.5 μM wild-type (WT) Coro1C or the following mutants: R28D; R28D/E379K; R28D/D431K; R28D/E438K; K418A, K419A/K427A, K428A (2xKA); K418E, K419E/K427E, K428E (2×KE); R28D/2xKA; or R28D/2xKE. Pellet (P) and supernatant (S) fractions were separated by SDS/PAGE. Representative Coomassie Blue-stained gel is from at least three independent experiments. (C) Table summarizing actin cosedimentation results from (B). Coro1C–R28D (D) or Coro1C–2xKE (E) (0.8 μM) was incubated with 0.3 μM actin followed by incubation with increasing concentrations of Coro1B. Actin cosedimentation was performed and pellet fractions were separated by SDS/PAGE. Representative Coomassie Blue-stained gels are from four independent experiments. Quantification is shown as amount of coronin bound to F-actin, which was normalized to the amount of actin in the pellet. Results are means±S.E.M. from the indicated number of experiments (n). Asterisk indicates statistical significance compared with initial concentration of coronin by one-way ANOVA; *P<0.05. In all gels, molecular mass markers are shown in kDa on the left-hand side.