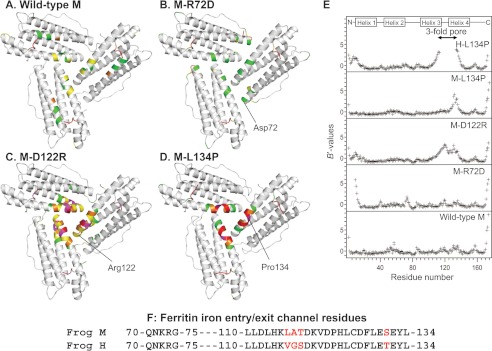
FIGURE 2.
Changing ion channel residues selectively affects main-chain flexibility. A–D, view from the internal surface of ferritin protein cages around the ion channel exits. The same sections of three helices form the ion channels around the 3-fold symmetry axes of the cage. The colors indicate the distribution of normalized B value (B′ value) for Cα atoms: white, B′ < 0.5; green, 0.5 < B′ < 1.0; yellow, 1.0 < B′ < 1.5; magenta, 1.5 < B′ < 2.0, red, 2.0 < B′. A, wild-type frog M (PDB code 3KA3); B, frog M-L134P (PDB 3SHX); C, frog M-D122R (PDB code 3SH6); D, frog M-R72D (PDB code 3SE1). E, B′ values of the Cα atoms for frog M ferritins and frog H-L134P (PDB code 1BG7) from (11); wild-type H and M ferritin isoforms share 85% sequence identity. F, amino acid sequence comparisons. The 3-fold channel sequences frog M and H ferritins are compared. Red type, difference in H and M ion channel sequence.