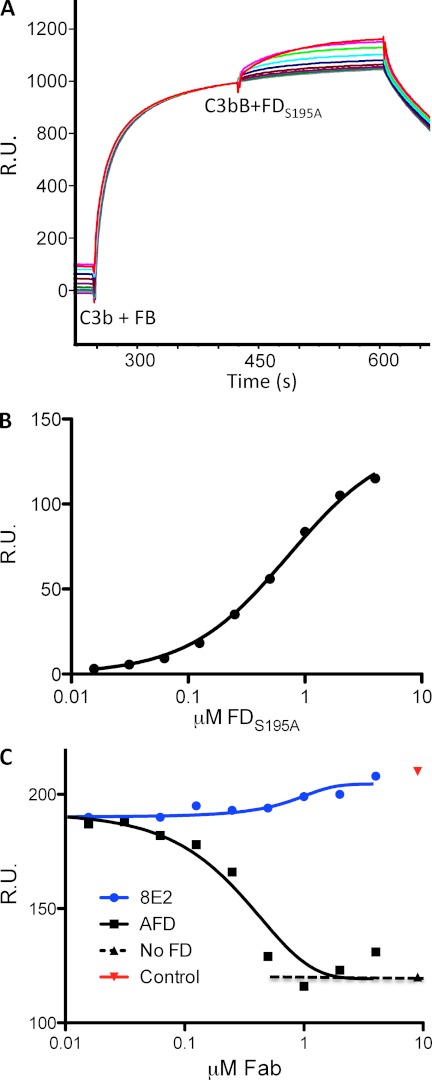
FIGURE 2.
AFD blocks binding of FD to C3bB proconvertase. A, binding of FD to a preformed C3bB complex as shown by surface plasmon resonance. A fixed concentration of FB (1 μm) was injected over immobilized C3b, followed by a dilution series of enzymatically inactive FD (FD(S195A), 2-fold dilution series of 4.0 μm to 15.6 nm). B, the binding affinity of FD for C3bB (720 nm) was measured by detecting the response (y axis) to increasing concentrations of FD(S195A) and a fixed concentration of FB (1 μm) flowed over immobilized C3b. Steady-state analysis of the binding data was used to derive dissociation constants for FD binding to C3bB. C, AFD blocked binding of FD to the C3bB complex. Fixed concentrations of FB (1 μm) and FD(S195A) (1 μm) and increasing concentrations of AFD or 8E2 were injected over immobilized C3b. The IC50 value averaged over three separate experiments is 0.43 ± 0.20 μm (mean ± S.D.) with full blockade at 0.82 ± 0.18 μm, which indicates that AFD blocks proconvertase activation at an ∼1:1 molar ratio of AFD to FD. R.U., resonance units.