FIGURE 4.
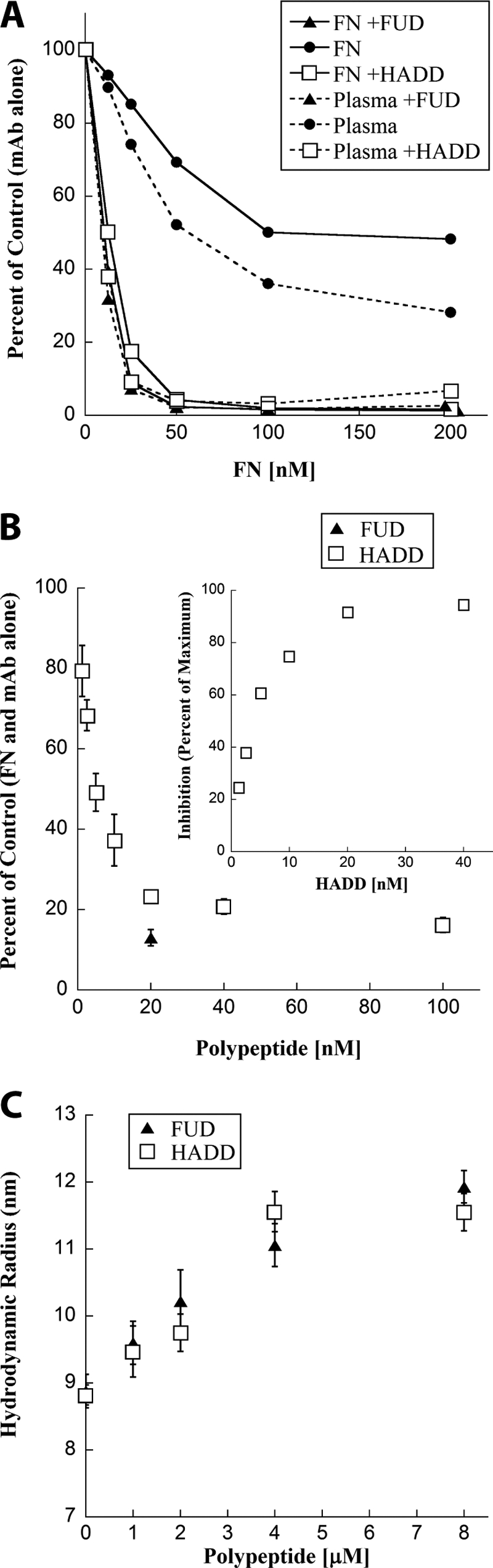
HADD is similar to FUD in exposing the mAbIII-10 epitope of purified FN or FN in plasma and expanding purified FN. A, effect of HADD or FUD on the exposure of the mAbIII-10 epitope in purified FN and FN in plasma as determined by competitive ELISA. Purified FNs (solid lines) or FNs in diluted plasma (dotted lines), 233 nm, were incubated without (●) or with 580 nm FUD (▴) or HADD (□) for 30 min. Prior to the assay, the concentration of FN in neat plasma was found to be 0.65 mg/ml (2600 nm) by competition ELISA with a mAb that is not conformation-sensitive. The six samples were then diluted to the indicated FN concentrations; mAbIII-10 was added, and competition by soluble FN for mAbIII-10 binding to coated FN was determined. Data are expressed as percent of mAbIII-10 binding alone (no FN or polypeptide added) and are representative of two experiments. B, ELISA of competition of binding of mAbIII-10 to coated FN by 20 nm soluble FN alone or preincubated with the indicated concentrations of HADD (□) or with 20 nm FUD (▴). Values are expressed relative to 20 nm soluble FN with mAbIII-10 but no polypeptide and represent mean ± S.D. of three experiments. The inset replots the HADD titration in comparison with the maximum inhibition found with 40 nm HADD. C, HADD (□) or FUD (▴) were titrated into FN solution separately, and the hydrodynamic radius of 4 μm FN or 4 μm FN plus the indicated concentration of polypeptide was calculated. Measurements were performed at 25 °C in 20 mm Tris, 100 mm sodium chloride, pH 7.4. Error bars indicate the standard deviation of six measurements on each sample. The experiment was repeated twice with the same result.
