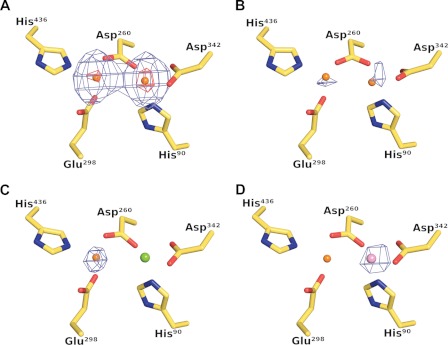
FIGURE 3.
Crystallographic identification of active site metals. The left and right metal-binding sites are referred to as site A and site B, respectively. A, Bijvoet-difference Fourier maps calculated using data collected at the zinc K absorption peak and contoured at the 5 r.m.s.d. (blue mesh) and 25 r.m.s.d. (red mesh) levels show strong anomalous scattering at the two metal sites, whereas the corresponding map calculated using pre-edge data shows only weak anomalous scattering at both sites as shown in B, thus demonstrating the presence of zinc at both sites in the as-isolated protein. C, anomalous scattering is only observed at site A in crystals grown in the presence of magnesium, suggesting that magnesium occupies site B in this crystal form. D, Bijvoet-difference Fourier map calculated using manganese K absorption peak data collected from a crystal composed of manganese-activated DNPEP. A single 9 r.m.s.d. peak is found at site B demonstrating that manganese can substitute for zinc at site B leading to an enhancement of enzymatic activity.