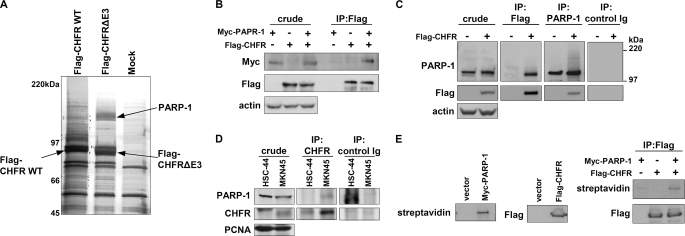
FIGURE 1.
CHFR interacts with PARP-1. A, HEK-293T cells were transiently transfected with FLAG-CHFR WT or FLAG-CHFR-deleted E3 activity (ΔE3) expression vectors or mock-transfected, and cell lysates were immunoprecipitated with anti-FLAG resin. Proteins that coimmunoprecipitated with FLAG-CHFR were separated by SDS-PAGE and negative gel staining and subjected to LC-MS/MS analysis. PARP-1 was identified as a CHFR-interacting protein. B, HCT116 cells, which do not express endogenous CHFR, were transfected with FLAG-CHFR and Myc-PARP-1 expression vectors. The cell lysates were immunoprecipitated (IP) with an anti-FLAG antibody, and Myc-PARP-1 was coimmunoprecipitated. Immunoblotting was performed with antibodies against each of the indicated proteins. C, HeLa cells, which do not express endogenous CHFR, were transfected with FLAG-CHFR expression vectors. The cell lysates were immunoprecipitated using anti-FLAG or anti-PARP-1 antibodies or control Ig. Immunoblotting was performed with antibodies against the indicated proteins. Endogenous PARP-1 and FLAG-CHFR were coimmunoprecipitated with FLAG-CHFR and PARP-1, respectively. D, the nuclear extracts form MKN45 and HSC-44cells, which express or do not express endogenous CHFR, respectively, were immunoprecipitated using anti-CHFR antibodies or control Ig. Immunoblotting was performed with antibodies against the indicated proteins. E, FLAG-CHFR was purified from HEK-293T cells with an anti-FLAG antibody and incubated with the biotinylated PARP-1 generated by in vitro translation. The complexes were thoroughly washed and analyzed by blotting with an anti-FLAG antibody or streptavidin-HRP.