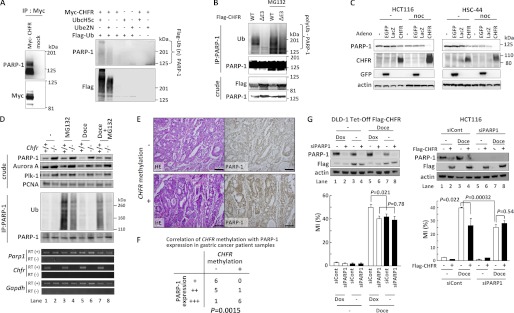
FIGURE 3.
CHFR polyubiquitinates PARP-1 and caused cell cycle arrest via PARP-1 degradation. A, HEK-293T cells were mock-transfected or transfected with Myc-CHFR expression vectors, and PARP-1 and Myc-CHFR complexes were coimmunoprecipitated (IP) with an anti-Myc antibody (left panel). The resins were thoroughly washed, and in vitro ubiquitination assays were performed (right panel). Immunoblotting was performed with antibodies against each of the indicated proteins. B, HCT116 cells were transfected with FLAG-CHFR or FLAG-CHFR ΔE3 expression vectors. After transfection for 24 h, the cells were treated with or without 10 μm MG132 for 4 h. The cell lysates were immunoprecipitated with an anti-PARP-1 antibody, and the precipitates were examined by immunoblotting with anti-ubiquitin (Ub), anti-PARP-1, or anti-FLAG antibodies. C, HCT116 and HSC44 cells, which do not express endogenous CHFR, were infected with adenoviruses expressing CHFR (CHFR WT), enhanced green fluorescent protein (EGFP), or LacZ. After infection for 64 h, the cells were treated with 0.5 μg/ml of nocodazole (noc) or DMSO (-) for 27 h. The cell lysates were examined by immunoblotting with the indicated antibodies. D, Chfr+/+ or Chfr−/− MEFs were treated with 1 μm docetaxel for 12 h, and 10 μm MG132 or DMSO was added following 4 h of incubation. Nuclear extractions were collected using the nuclear extract kit (Active Motif, Santa Clara, CA) and subjected to immunoprecipitation and immunoblotting with antibodies against each of the indicated proteins (top panel). RT-PCR was also performed (bottom panel). E, primary gastric cancer samples were stained using an anti-PARP-1 antibody. CHFR methylation status was analyzed as described under “Experimental Procedures.” Magnification ×10. Scale bar = 100 μm. F, statistical analysis of CHFR methylation status and PARP-1 expression levels from 19 primary gastric cancer samples. The Wilcoxon rank test was used to determine whether there was an association between the CHFR methylation status and PARP-1 expression. The p value is indicated. G, DLD-1 Tet-Off cells inducibly expressing FLAG-CHFR (DLD-1 Tet-Off FLAG-CHFR) were cultured with or without 0.1 μg/ml of doxycycline (Dox) for 24 h and transfected with a mixture of three siRNAs targeting PARP-1 (siPARP1) or control oligonucleotides (siCont). After transfection for 4 h, the cells were cultured with or without 0.1 μg/ml of doxycycline for 22 h following treatment with 0.5 μm docetaxel (Doce) for 16 h, and the MI was determined (left panel, bottom). HCT116 cells with stably knocked down PARP-1 (HCT116 siPARP1) or control (HCT116 siCont) cells were mock-transfected or transfected with FLAG-CHFR expression vectors. Twenty-four hours after transfection, the cells were treated with 0.5 μm docetaxel for 16 h, and the MI was determined (right panel, bottom). Experiments were performed in triplicate. The mean ± S.D. is indicated by bars and brackets, respectively. p values were calculated using Student's t test. The cell lysates were examined by immunoblotting with each of antibodies against the indicated proteins (top panel).