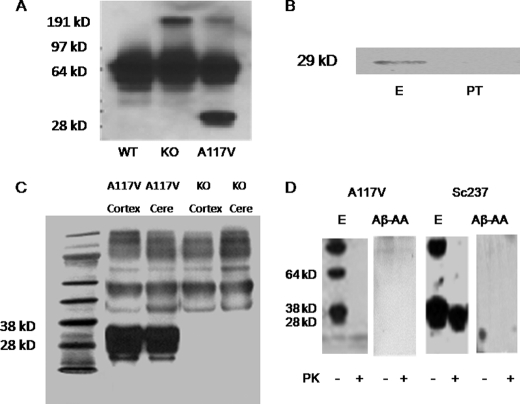
FIGURE 2.
Characterization of PrP-AA specificity for PrP. A, A117V transgenic mice, but not wild-type (WT) nor PNRP knock-out (KO) mice, were shown to express the PrP protein, which was detectable in brain homogenates using the murine monoclonal antibody 3F4. B, visualization of PrP(A117V) in brain homogenates (500 μg protein) of transgenic mice by immunoprecipitation with purified PrP-AA (E) or PT. Immunoprecipitated complexes were subjected to Western blot analysis with 3F4 antibody. C, purified PrP-AA recognized the PrP protein in Western blots of brain cortex and cerebellar (Cere) homogenates of A117V transgenic mice but not KO mice. Although, multiple bands were observed with overexposure, the strongest signal corresponded to the approximately band of 29 kDa PrP (A117V) observed in PrP(A117V) transgenic mice. D, Western blot analysis of immunoprecipitates from brain homogenates (1 mg transgenic mouse cerebellum and 10 mg Sc237 hamster brain) pretreated with or without proteinase K using PrP-AA or autoantibodies against Aβ. An anti-PrP antibody 6D11 which detects both mouse and hamster PrP, was used for detecting antibody. Numbers adjacent to horizontal lines indicate positions of molecular mass markers (kDa). 10 μl samples were loaded in each lane. Purified PrP-AA recognized both PrP and PK-resistant PrPSc (27–30kDa). Autoantibodies against Aβ did not recognize PrP nor PK-resistant PrPSc (27–30kDa). The photo was selected from a single representative experiment that was repeated three times with similar results. PT, pass-through IgG depleted of PrP-AA. Aβ-AA, autoantibodies against Aβ