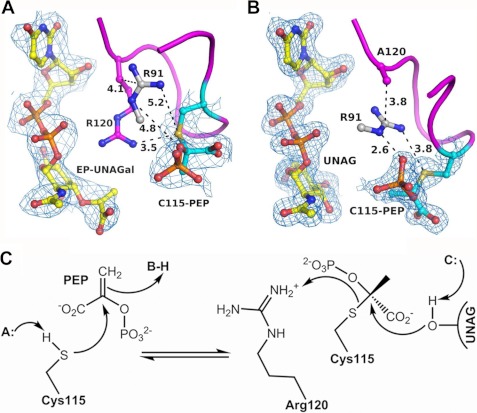
FIGURE 5.
The covalent adduct forms in vitro during the reaction of free MurA with UNAG and UNAGal. A, co-crystallization of free enzyme with UNAGal and PEP yielded EP-UNAGal and PEP covalently attached to Cys-115 (V), suggesting a single turnover and activation of PEP during the catalytic cycle. B, The Cys-115-PEP adduct also formed upon incubation of the R120A mutant enzyme with UNAG (II), suggesting that the guanidinium group of Arg-120 serves as proton donor in the transfer of PEP from Cys-115 to UNAG. C, in this mechanism the nucleophilic attack of the sulfanion on the C2 atom of PEP leads to formation of the thioketal, and the transfer of PEP to the target hydroxyl of UNAG is facilitated by protonation of Cys-115 by Arg-120. Shown as blue mesh is the 2 Fo − Fc electron density at 2.8 Å and 1.9 resolution, contoured at 1σ.