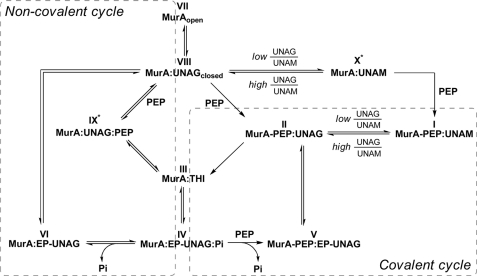
FIGURE 8.
Reaction mechanism of E. cloacae MurA according to the various enzyme states determined by crystallography. Shown are the covalent (right) and non-covalent (left) reaction cycles of MurA, which are interconnected via complexes III and IV. The structure identifiers are the same as in Table 1. Cellular MurA exists predominantly in the dormant complex (I), and the covalent reaction cycle may proceed as shown in Fig. 7. In vitro, the open unliganded enzyme (VII) interacts first with UNAG to form the binary complex (VIII). PEP may then react in a rapidly reversible manner to yield the ternary substrate complex (IX) or covalently with Cys-115 to yield complex II. Both complexes II and IX are catalytically competent and give rise to the THI (III). The product complex IV may release phosphate to yield complex VI followed by exchange with UNAG to revert to complex VIII, which completes the non-covalent cycle (VIII, IX, III, IV, and VI). It is possible that dormant complex I releases bound PEP in a single turnover reaction from II to III and that subsequent reactions proceed through the non-covalent cycle. With decreasing UNAG/UNAM ratios, a rapidly reversible complex with UNAM (X) may form to which PEP binds covalently and yields the dormant complex. IX* and X* denote that the crystal structures have not yet been determined.