FIGURE 2.
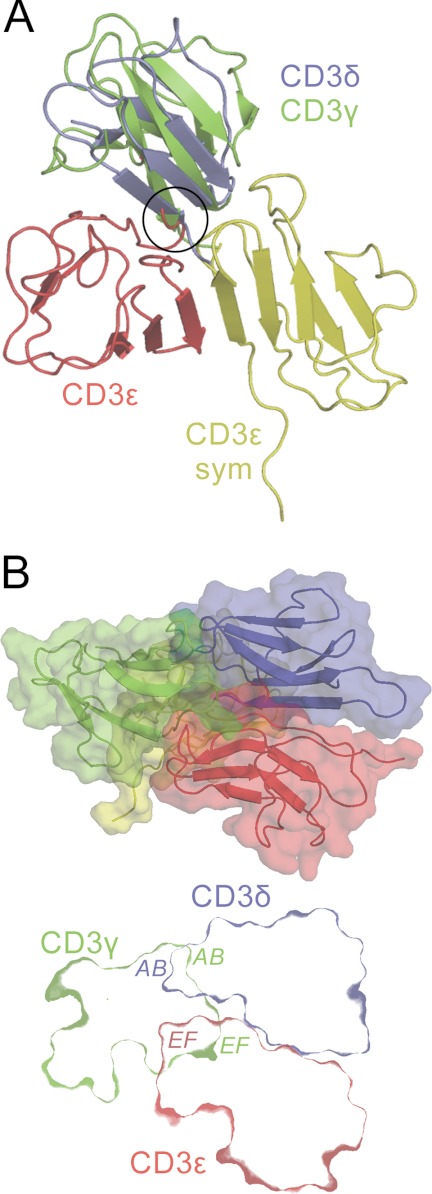
The CD3ϵ crystallographic homodimer is unlikely to mediate CD3 heterotetramerization. A, the crystallographic CD3ϵ homodimer is shown in red and yellow, with CD3δ (blue; Protein Data Bank code 1XMW) and CD3γ (green; Protein Data Bank code 1JBJ) shown docked with the red CD3ϵ monomer in the positions that CD3δ and CD3γ occupy in the NMR structures of the CD3ϵδ and CD3ϵγ heterodimers (20, 21). Prior heterodimerization of CD3ϵ with either CD3γ or CD3δ creates a clash (circled) that prevents the formation of the small homodimerization interface involving the C-terminal loops of CD3ϵ (box (ii) in Fig. 1B). B, upper panel, a putative heterotetrameric CD3 complex, with CD3δ (blue) and CD3γ (green) each docked with one of the CD3ϵ monomers in the CD3ϵ homodimer (red and yellow), in the manner observed in the NMR structures of the CD3ϵδ and CD3ϵγ heterodimers. Most of the yellow CD3ϵ monomer is hidden behind CD3γ. Lower panel, a section through the middle of the heterotetramer revealing the positions of the subunit surfaces; the section does not incorporate any part of the second (yellow) CD3ϵ monomer. Subunit regions that clash are labeled. These clashes would likely prevent formation of the heterotetramer in vivo. See also supplemental Fig. S1.