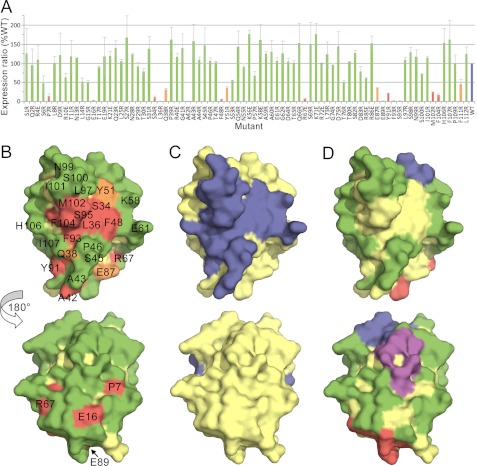
FIGURE 4.
Identification of surface-exposed and buried residues in the human CD8αα homodimer. A, histogram showing relative surface expression levels of drastically mutated, lentiviral expressed, HA-tagged CD8 proteins. The surface expression ratio is calculated as the median fluorescence of anti-HA antibody binding divided by the median fluorescence of IRES-encoded GFP (as an indicator of the efficiency of viral transduction), each measured by FACS. The surface expression ratio is normalized against the value obtained for the wild-type protein in the same experiment, and the average values for at least two experiments, expressed as percentages, are shown along with standard errors of the mean. The bars are colored according to whether mutation of the indicated residue reduced CD8 expression by more than 85% (red), by 60–85% (orange), or by less than 60% (green) versus wild-type HA-tagged CD8 expression (blue). B–D, the surface of CD8α (from Protein Data Bank code 1AKJ) (46) is shown. In each panel, the upper view looks onto the GFCC′C″ face, and the lower view is rotated 180° about the vertical axis (hence looking onto the DEBA sheet). In B, mutated residues are colored as described in A. In C, residues shown by crystallographic analysis to mediate CD8αα homodimerization are colored blue. In D, residues whose drastic mutation affects cell surface expression are colored yellow, and the remaining residues are colored according to whether they drastically reduce (>85%) the binding of the anti-CD8 mAbs DK25 (blue), SK1 (purple), or OKT8 (red) or have no effect on expression or antibody binding (green). See also supplemental Fig. S3.