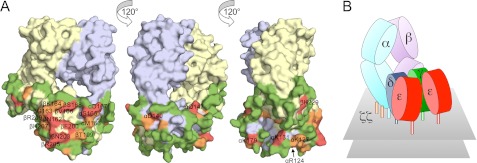
FIGURE 6.
Surface mutation of TCRαβ and model for the TCR-CD3 complex. A, three views of the surface of the TCRαβ heterodimer (from Protein Data Bank code 1OGA (71)), each related by a 120° rotation about the vertical axis. The α chain is colored blue-gray, and the β chain is yellow. Mutated residues are colored according to whether their mutation reduces TCR expression by more than 85% (red), by 60–85% (orange), or by less than 60% (green) versus wild-type TCR expression. B, cartoon illustration of the proposed quaternary arrangement of extracellular domains within the TCR-CD3 complex based on all of the mutagenesis data presented here. It remains unclear whether both CD3γ and δ contact the TCRαβ heterodimer or whether one (most likely CD3δ (17)) forms the major contact and stabilizes the association of the other in the complex in the absence of direct contacts with TCRαβ. See also supplemental Figs. S5 and S6.