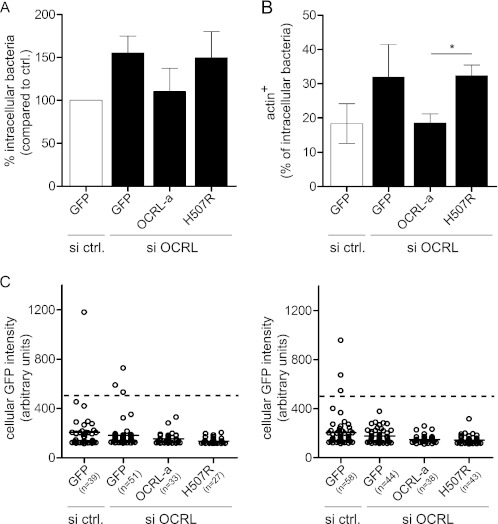
FIGURE 6.
OCRL phosphatase function is responsible for restricting L. monocytogenes invasion and for promoting actin depolymerization on bacterial vacuoles. A, one set of HeLa cells was transfected with control siRNA and a plasmid coding for GFP, whereas a second set of HeLa cells was transfected with anti-OCRL siRNA and plasmids coding for GFP, siRNA-resistant pEGFP-OCRL-a, or pEGFP-OCRL-a phosphatase-dead (H507R). After 1 h of infection with L. monocytogenes P14.PrfA*, cells were processed for immuno-fluorescence, and invasion was quantified by scoring extracellular versus total cell-associated bacteria. Values represent the averages ± S.E. of 3 independent experiments. si ctrl., siRNA control. B, transfections were performed as described in A, and after 15 min of infection with L. monocytogenes P14.PrfA*, cells were processed for immuno-fluorescence microscopy to quantify the percentage of intracellular bacteria associated to actin (labeled by fluorescent phalloidin). Values represent the averages ± S.E. of 3 independent experiments. (Student's t test: *, p < 0.05.) C, the GFP fluorescence intensity of cells chosen for quantifications in A and B was measured using ImageJ. Circles represent cells, and horizontal lines represent the average of all cells of the given condition. For quantification, low to medium GFP-expressing cells with a GFP intensity lower than 500 units (dashed line) were considered.