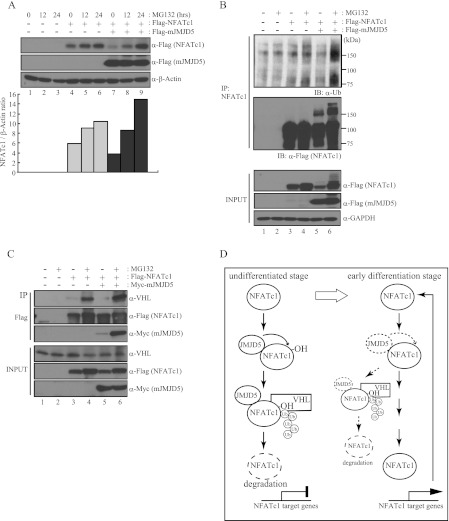
FIGURE 6.
JMJD5 induced ubiquitin-mediated proteasomal degradation of NFATc1. A, HEK293T cells were co-transfected with FLAG-NFATc1 and FLAG-mJMJD5 and then treated with MG132 (10 μm) for the indicated times. After 48 h of transfection, total cell lysates were prepared and analyzed by immunoblotting with anti-FLAG antibody. β-Actin was used as a loading control for immunoblotting. The signals obtained from the immunoblotting were quantified with the ImageJ program. B and C, HEK293T cells were co-transfected with FLAG-NFATc1 and Myc-mJMJD5 and then treated with MG132 (10 μm) for 16 h. After 48 h of transfection, total cell lysates were prepared and subjected to immunoprecipitation (IP) with an anti-NFATc1 antibody (B) or anti-FLAG M2-agarose (C). Bound proteins were detected by immunoblotting with the indicated antibodies. GAPDH was used as loading control for immunoblotting. D, suggested model.