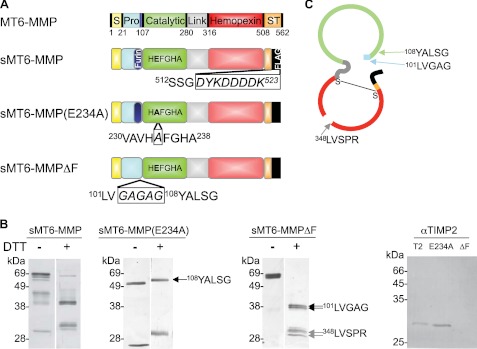
FIGURE 1.
Preparation of MT6-MMP. A, domain structures of MT6-MMP proteins generated here by site-directed mutagenesis indicating the signal peptide (S; yellow), propeptide (Pro; blue) with a furin cleavage site (navy), catalytic domain (green), flexible linker (gray), hemopexin domain (red), and stalk and hydrophobic tail for glycosylphosphatidylinositol anchoring (ST; orange). The stalk was truncated, and a FLAG tag (black) was appended to create an sMT6-MMP construct. An additional mutation to sMT6-MMP was made to remove the furin cleavage site (sMT6-MMPΔF). Alternatively, a further mutation to sMT6-MMP was made to exchange the catalytic Glu residue to Ala, resulting in the catalytically inactive sMT6-MMP(E234A) protein. B, purified sMT6-MMP, sMT6-MMP(E234A), and sMT6-MMPΔF proteins were visualized under non-reducing (−DTT) or reducing conditions (+DTT) on silver-stained 15% SDS-PAGE. The differences in protein migration in these two conditions are due to differences arising from the effects of disulfide bonding under non-reduced compared with reduced conditions. In comparing these conditions in the wild type and inactive mutants, the different electrophoretic migrations of protein in the two preparations relate to the presence or absence of autolytic cleavage within the hemopexin domain. Thus, in the absence of catalytic activity in the sMT6-MMP(E234A) recombinant form, no autocatalytic cleavage occurs in the hemopexin domain, resulting in electrophoretic migration under nonreducing conditions consistent with a disulfide bond-constrained domain that migrates faster than when reduced or when cleaved and non-reduced in the wild type sMT6-MMP form. Edman sequencing of sMT6-MMP(E234A) showed the N terminus to be Tyr108 (arrow). Sequencing showed the N terminus of sMT6-MMPΔF to be Leu101 (double arrows) with a cleavage product occurring within the hemopexin domain at Leu348 (double gray arrows). Western blot analysis of purified mTIMP2 (T2), sMT6-MMP(E234A) (E234A), and sMT6-MMPΔF (ΔF) with a primary antibody to TIMP2 indicates the presence of TIMP2 in the sMT6-MMP(E234A) but not sMT6-MMPΔF preparation. C, schematic of sMT6-MMPΔF indicating the seven amino acids remaining at the N terminus upstream of the catalytic domain and cleavage point at Leu348 within the hemopexin domain.