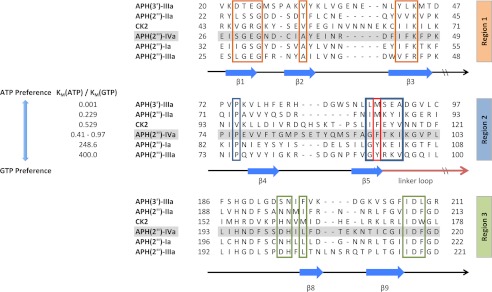
FIGURE 3.
Partial multiple sequence alignment of selected APH enzymes and CK2. The aligned enzymes are ordered based on increasing preference for GTP as the second substrate. Secondary structural elements are indicated below the alignment. Residues forming the nucleoside-binding site in APH(2″)-IVa are separated into three regions. Phe-95 of APH(2″)-IVa and its corresponding amino acids in the other enzymes are highlighted by the red box. The alignment for APH(2″) enzymes was created with Clustal Omega (32). APH(3′)-IIIa and CK2 were aligned based on a manual structural alignment between representative structures (PDB accession numbers 1L8T and 1LP4) and the APH(2″)-IVa-adenosine complex. The kinetic parameters for the six enzymes were taken from literature (3, 33, 34). The Km(ATP)/Km(GTP) ratio for APH(2″)-IVa varies among three independent studies due to small differences in specific experimental conditions (3, 13).