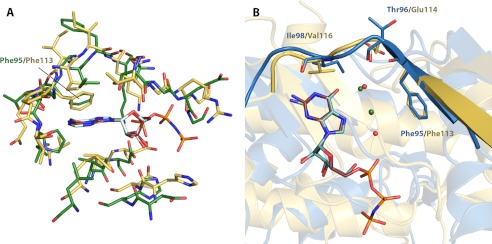
FIGURE 5.
Structural comparison between nucleoside-bound complexes of APH(2″)-IVa and CK2. A, structural superposition of residues forming the nucleoside-binding pocket of AMPPNP-bound CK2α (yellow with red ligand) onto those of adenosine-bound APH(2″)-IVa (green with light cyan ligand). Despite significant discrepancies in the overall protein structure, this region shows strong structural conservation. B, structural superposition of GMPPNP-bound CK2α structure (yellow with red ligand) onto guanosine-bound APH(2″)-IVa (blue with dark cyan ligand). The conformation of the interdomain linker, highlighted in graphic representation, and the position of key residues, shown in stick representation, are conserved. Also conserved between the two structures is a solvent network, consisting of two water molecules for CK2 (green spheres) and three water molecules for APH(2″)-IVa (red spheres, with one overlapping and occluded by a green sphere).