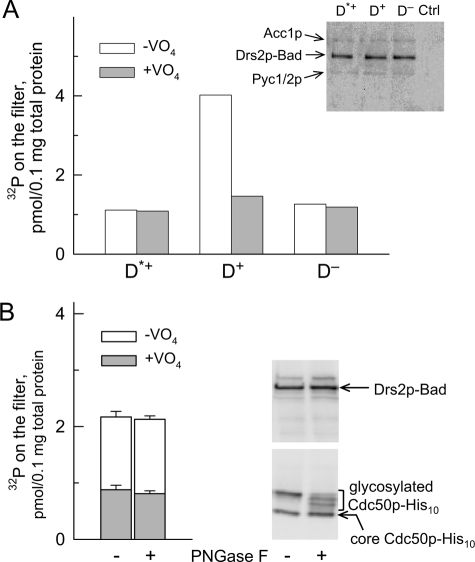
FIGURE 3.
Co-expression of Cdc50p, but not its full glycosylation, is required for phosphorylation (from [γ-32P]ATP) of Drs2p-Bad expressed in P3 membranes. A, P3 membranes containing either Drs2p mutated at the catalytic site and co-expressed with Cdc50p (Drs2pD560N, D*+), wild-type Drs2p co-expressed with Cdc50p (D+), or wild-type Drs2p expressed alone (i.e. in the presence of endogenous Cdc50p only (D−)) were used. Samples (200 μl) were incubated on ice at 0.5 mg of total protein/ml in buffer A supplemented with 100 μm Ca2+ in the absence or presence of 1 mm orthovanadate. At time 0, phosphorylation was triggered by addition of a final concentration of 2 μm [γ-32P]ATP; after 25–30 s, this was followed by acid quenching and filtration. This graph shows one representative experiment of several independent experiments with similar results. Inset, after loading 0.25 μg of total protein for SDS-PAGE, Drs2p-Bad was detected using a biotin probe. In the range of molecular masses illustrated, the probe also weakly detected the biotinylated yeast proteins Acc1p (∼250 kDa) and Pyc1/2p (∼130 kDa). Ctrl, yeast membranes that were not transformed with DRS2- or CDC50-containing plasmid. B, D+ P3 membranes suspended at 2 mg/ml in SB containing 1 mg/ml DDM were incubated on ice for 15 min in the absence or presence of 10 μg/ml peptide-N-glycosidase F (PNGase F) (i.e. 20,000 units/ml). Membranes were subsequently diluted to 0.5 mg/ml in buffer A supplemented with 0.1 mm Ca2+ and 1 mm EGTA, and the ability of these samples to become phosphorylated was measured after addition of 0.5 μm [γ-32P]ATP in the absence (open bars) or presence (gray bars) of 1 mm orthovanadate. Data are presented as the mean ±S.D. (error bars) of duplicates. 1.5 μg of total protein was loaded for Western blot analysis. Detection of Drs2p was performed using a biotin probe, and detection of Cdc50p was performed using a His probe (gels on the right).