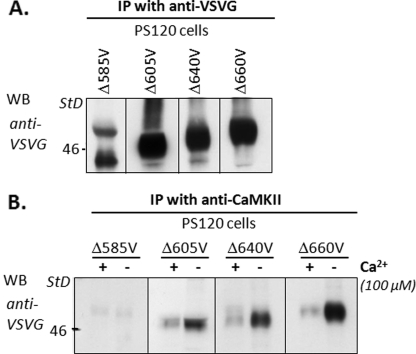
FIGURE 8.
CaMKII binds in vivo to NHE3 between aa 586 and 605. A, immunoprecipitation (IP) of a series of NHE3 C-terminal truncation mutants truncated at aa 585, 605, 640, 660, and 690 (NHE3/Δ585V, NHE3/Δ605V, NHE3/Δ640V, NHE3/Δ660V, and NHE3/Δ690V, respectively) were identified with anti-VSVG polyclonal antibodies (data not shown for Δ690). B, cell lysates with NHE3 C-terminal truncation mutants were immunoprecipitated with anti-CaMKIIγ antibody in the presence (100 μm) and absence (1 mm EGTA) of Ca2+, and coprecipitated NHE3 truncation mutants were visualized with anti-VSVG antibodies. CaMKII co-immunoprecipitated all tested NHE3 C-terminal truncation mutants except NHE3/Δ585V. NHE3 mutants were all co-precipitated in the presence and absence of Ca2+ with less binding when Ca2+ was elevated.