Table 1.
Experimental and theoretical kinetic isotope effects
| Peptide substrates |
Experimental KIEs*† |
Theoretical structures and KIE predictions |
|||||||
| Heavy |
Light |
Native |
I84V |
8 |
9 |
10 |
11 |
12 |
13 |
| Ac-SQN[1-14C]F‡PVV-NH2 | [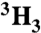 ]Ac-SQNF‡PVV-NH2 ]Ac-SQNF‡PVV-NH2
|
1.029(3) | 1.025(5) | 1.073 | 1.017 | 1.020 | 1.019 | 1.057 | 1.025 |
[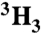 ]Ac-SQNF‡[15N]PVV-NH2 ]Ac-SQNF‡[15N]PVV-NH2
|
[14C]Ac-SQNF‡PVV-NH2 | 0.987(3) | 0.989(3) | 1.000 | 1.002 | 0.996 | 0.992 | 1.015 | 0.995 |
[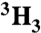 ]Ac-SQN[18O]F‡PVV-NH2 ]Ac-SQN[18O]F‡PVV-NH2
|
[14C]Ac-SQNF‡PVV-NH2 | 0.993(3) | 0.999(3) | 0.991 | 0.995 | 0.994 | 0.994 | 0.996 | 0.995 |
| Ac-SQN[α-3H]F‡PVV-NH2 | [14C]Ac-SQNF‡PVV-NH2 | 0.968(1) | 0.976(1) | 0.938 | 0.922 | 0.919 | 0.908 | 0.874 | 0.917 |
KIE, kinetic isotope effect.
*Experimental errors are shown in parentheses (x10-3) following the KIE values and reflect the standard error of the mean resulting from n ≥ 18 independent measurements.
†KIEs obtained using the [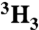 ]-Ac remote radiolabel are corrected for a 3H effect that was measured to be 0.974(2) and 0.976(2) for the native and I84V enzyme, respectively.
]-Ac remote radiolabel are corrected for a 3H effect that was measured to be 0.974(2) and 0.976(2) for the native and I84V enzyme, respectively.
‡Denotes the location of the scissile bond.
