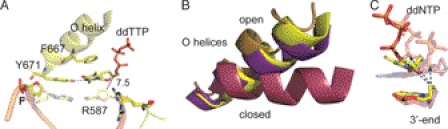
FIGURE 3.
Structure of KlenTaqF-T (yellow). A, stabilization network of ddTTP. The amino acid side chains Arg-587, Phe-667, and Tyr-671 are labeled. Gray and black dashed lines indicate hydrogen-bonding interactions and distance (Å), respectively. B, the O helices from the superimposition of KlenTaqF-T (yellow), KlenTaqF-A (purple), KlenTaqA-T (magenta), and KlenTaqbinary (gold) are highlighted. C, the incoming ddNTPs of KlenTaqF-T (yellow), KlenTaqF-A (purple), and KlenTaqA-T (berry) and the respective 3′-primer terminus are shown. The arrow indicates the displacement of the α-phosphate regarding the 3′-primer terminus.