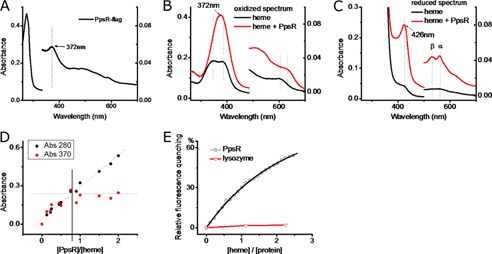
FIGURE 1.
PpsR binds heme. A, UV-visible spectrum of purified PpsR-FLAG from R. sphaeroides. B, UV-visible spectrum of heme and PpsR-heme (the spectrum of PpsR is subtracted) under oxidizing conditions. 5 μm heme was incubated with 10 μm PpsR for at least 20 min before the spectrum was taken. C, UV-visible spectrum of heme and PpsR-heme under reducing conditions. D, titration of PpsR in to heme. The change of the absorbance at 280 nm indicates the change of protein concentration whereas the change of Soret peaks at 370 nm indicating the formation of PpsR-heme complex. E, binding constant of PpsR-heme interaction. Heme was titrated into 1 μm PpsR, with at least 5 min of incubation time between each step. The data were fitted with one-to-one binding model. The same settings were applied to 1 μm lysozyme.