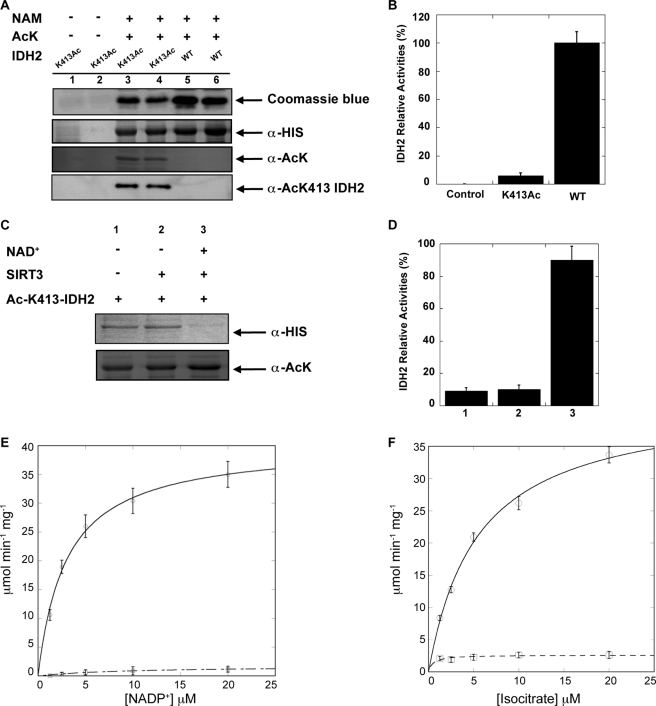
FIGURE 2.
A, in vivo site-specific incorporation of acetylated Lys-413 in IDH2. IDH2 and IDH2K413Ac were recombinantly expressed and purified and analyzed by 12% SDS-PAGE or detected in total lysates by Western blot with an anti-His6 antibody or anti-pan acetyllysine antibody. NAM, nicotinamide; AcK, acetyllysine. B, relative IDH2 activity assays using purified IDH2 and IDH2K413Ac. C and D, SIRT3 specifically deacetylates IDH2K413Ac and rescues activity. C, recombinant IDH2K413Ac from A was incubated with purified recombinant SIRT3 with or without NAD+ at 37 °C for 1 h. Acetylation status was assessed by Western blotting with anti-acetyllysine antibody. An anti-His Western blot shows that equivalent IDH2 protein levels were used. D, IDH2 activity assays were performed using the corresponding IDH2K413Ac or unacetylated IDH2 from C. E and F, steady-state kinetic analysis of wild-type IDH2 and IDH2K413Ac. Recombinant proteins were purified by nickel affinity chromatography, and kinetic assays were performed as described under “Experimental Procedures.” Comparison of wild-type IDH2 (open circles) and IDH2K413Ac (open squares) reveals that acetylation greatly affects Vmax and Vmax/Km. Data are means ± S.E.