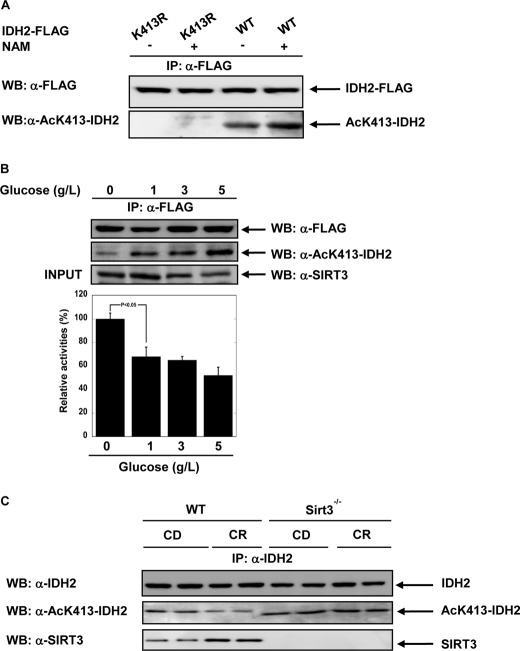
FIGURE 4.
A, in cells, IDH2 is acetylated on lysine 413. A specific acetyllysine 413 antibody was generated using the peptide SGAMT(AC)KDLAGC. HEK293 cells were co-transfected by pcDNA3-IDH2-FLAG and pcDNA3-IDH2K413R-FLAG. IDH2 proteins were immunoprecipitated (IP) using FLAG beads. IDH2 Lys-413 acetylation levels were detected using the site-specific antibody. WB, Western blotting; NAM, nicotinamide; AcK, acetyllysine. B, low glucose decreases acetylation level of IDH2 Lys-413 and increases IDH2 activity. IDH2-FLAG was overexpressed in HEK293 cells following treatment with varying glucose levels (5, 3, 1, and 0 g/liter) for 6 h. Cell lysates were resolved by SDS-PAGE and detected by Western blotting with anti-SIRT3 antibody. IDH2-FLAG was immunoprecipitated with anti-FLAG beads, and IDH2 activity was measured and normalized to IDH2 protein levels; quantifications of the amounts of IDH2 were performed by anti-FLAG antibody and anti-acetylated Lys-413 antibody from A. Error bars represent standard error measurement (S.E.) (n = 3), p < 0.05. C, Bottom and middle, Western blot analysis of SIRT3 (bottom) and levels of acetylated lysine 413 (middle) in liver from 5-month-old WT or Sirt3−/− mice fed either control diet (CD) or calorie-restricted diet (CR). Top, endogenous acetylated IDH2 was isolated by immunoprecipitation with anti-IDH2 antibody followed by Western blotting with anti-acetyllysine 413 IDH2 antibody (middle).