Background: Appropriate termination of photoresponse is critical for photoreceptors to achieve high temporal resolution and to prevent excessive Ca2+-induced cell toxicity.
Results: We isolated a novel Gαq mutant allele and revealed that metarhodopsin/Gq interaction affects Arr2-Rh1 binding.
Conclusion: Gq modulates the termination of phototransduction and prevents retinal degeneration.
Significance: Our study revealed the novel role of Gq in phototransduction deactivation and in retinal degeneration.
Keywords: Arrestin, G Protein-coupled Receptors (GPCRs), G Proteins, Phototransduction, Rhodopsin, Arr2-Rh1 Binding, Termination
Abstract
Appropriate termination of the phototransduction cascade is critical for photoreceptors to achieve high temporal resolution and to prevent excessive Ca2+-induced cell toxicity. Using a genetic screen to identify defective photoresponse mutants in Drosophila, we isolated and identified a novel Gαq mutant allele, which has defects in both activation and deactivation. We revealed that Gq modulates the termination of the light response and that metarhodopsin/Gq interaction affects subsequent arrestin-rhodopsin (Arr2-Rh1) binding, which mediates the deactivation of metarhodopsin. We further showed that the Gαq mutant undergoes light-dependent retinal degeneration, which is due to the slow accumulation of stable Arr2-Rh1 complexes. Our study revealed the roles of Gq in mediating photoresponse termination and in preventing retinal degeneration. This pathway may represent a general rapid feedback regulation of G protein-coupled receptor signaling.
Introduction
Heterotrimeric G proteins play pivotal roles in mediating extracellular signals from hormones, neurotransmitters, peptides, as well as sensory stimuli to intracellular signaling pathways (1, 2). In Drosophila photoreceptors, G proteins are essential for the activation of the phototransduction cascade (3, 4). Photon absorption leads to the photoisomerization of chromophores, resulting in the formation of activated metarhodopsin. In turn, metarhodopsin activates heterotrimeric G proteins and PLC.2 The activation of PLC leads to transient receptor potential and transient receptor potential-like channels opening and extracellular Ca2+ influx (5–8).
It is also critical for each step of the phototransduction cascade to be terminated appropriately, which is essential for the high temporal resolution of fly vision (9, 10). The most important step in phototransduction termination is the deactivation of metarhodopsin. During this step, arrestin (Arr2) plays an important role by displacing the Gq α subunit and allowing it to bind with rhodopsin (Rh1) (11, 12). Unlike other G protein-coupled receptors (GPCRs), the phosphorylation of fly rhodopsin is not required for its deactivation (13) but is essential for its endocytosis (11). In contrast, the dephosphorylation of rhodopsin by retinal degeneration C (RDGC) is essential for receptor deactivation (14). Ca2+ also plays critical roles in regulating the termination of the photoresponse in Drosophila (8, 15, 16). Several proteins that mediate this Ca2+-regulated termination have been identified, such as eye-specific protein kinase C (INAC), calmodulin, INAD, and myosin III (NINAC) (8, 17–20).
It has been shown that the loss of DGq leads to the slow termination of the light response (4). Here, we provide evidence that the metarhodopsin/Gq interaction affects subsequent Arr2-Rh1 binding, which mediates the deactivation of metarhodopsin. Our study has characterized the role of Gq in photoresponse termination. This pathway represents a general rapid feedback regulation in GPCR signaling.
EXPERIMENTAL PROCEDURES
Fly Genetics
The genotype of wild-type flies is cn,bw. sast mutants were generated with the chemical mutagen ethyl methanesulfonate (EMS) in cn,bw background, and all flies were put into cn,bw background to eliminate the effects of eye color. The mutant alleles used for each gene in this work are ninaE8, Gαq1, and norpA24. To avoid age-dependent retinal degeneration, all flies were reared at 22 °C in dark and examined when they were 2–3 days old. The Gαq mutant and p[hs::dGαq] transgenic fly were obtained from the C. Zuker laboratory, and all other flies used in this work were from the Bloomington Stock Center. The deficiency line Df(2R)Exel7121 was obtained from the Bloomington Stock Center and recombined into FRT 42D background. Genetic mosaics were induced by the FLP-FRT p[GMR-hid] technique with an ey-FLP driver to generate mitotic clones of a single genotype in the eye (21).
Generation of Transgenic Flies
To generate GqH212A transgenic flies, a mutate dGq cDNA, which mutate His212 to Ala, was subcloned into pUAST vector and then injected into w1118 flies. The transgene was subsequently crossed into the Gαq1 and Gαq961 mutant background, respectively. GqH212A protein was expressed by using eye-specific driver (p[gmr::GAL4]).
Electrophysiological Recordings
Electroretinogram (ERG) recordings were performed as described previously (22). Briefly, fly eyes were stimulated with 5-s light pulses (4000 lux). For each genotype and condition, >10 flies were examined. To quantitate the speed of response termination, the time required for half-recovery was measured, and the S.E. was calculated. t½ was defined as the time required for a half-recovery from the responses upon stimulation cessation. Prolonged depolarization afterpotentials were examined using the same setup except with different color filters and light intensities.
Relative light sensitivity measurement was carried out as described previously (23). For each genotype and condition, 10 flies were examined, and the relative sensitivities were averaged to obtain a mean. The S.E. were calculated and presented as error bars in the figures.
Intracellular recordings were performed as described previously (24). Briefly, a low resistance glass microelectrode filled with 2 m KCl was placed into a small hole on the compound eye. A reference electrode was filled with Ringer's solution and inside the eye at the retina layer. The microelectrode placed on the eye was gradually inserted into the opening until light-induced membrane depolarization was observed. The signal was amplified and recorded using a Warner IE210 Intracellular Electrometer.
Western Blotting
Fly heads were homogenized in SDS-sample buffer, and the proteins were fractionated by SDS-PAGE and transferred to PVDF membranes (Pall) in Tris-glycine buffer. The blots were probed with mouse anti-Rh1 antibodies (1:3000 dilution, Developmental Studies Hybridoma Bank), rabbit anti-Gβe antibodies (1:2000 dilution) (25), rabbit anti-Gαq antibodies (1:2000 dilution, Calbiochem), rabbit anti-N-Gαq antibodies (1:1000 dilution; Abcam), rabbit anti-Arr2 antibodies (1:1000 dilution), rabbit anti-INAD antibodies (1:1000 dilution). The blots were subsequently probed with either anti-rabbit, mouse IgG-peroxidase conjugate (GE Healthcare), and the signals were detected using ECL reagents (Amersham Biosciences).
EM and Immunolocalizations
Fly heads were immersed in 2.5% glutaraldehyde, 0.1 m sodium cacodylate (pH 7.2) at 4 °C for 12 h. After rinsing three times with 0.1 m sodium cacodylate, the fixed tissue was stained with 1% osmium tetroxide for 1 h at room temperature. A standard ethanol dehydration series was performed, and tissue was immersed in two 10-min washes of propylene oxide. The tissue was then embedded using standard procedures. For electron microscopy, thin sections (100 nm) were cut, collected on copper support grids, and stained with uranyl acetate followed by lead citrate staining. Micrographs were taken at 80 kV on Hitachi-7650.
For immunofluorescence staining of Rh1 and Arr2, fly heads were fixed with 4% paraformaldehyde in PBS, dehydrated with acetone, and embedded in LR White resin. One-micrometer sections were cut and double stained with anti-Rh1 antibody, 4C5 (1:100, DSHB), and anti-Arr2 antibody (1:400).
Arr2 Binding Assays
Arr2 binding assays were performed as described previously with a minor modification (26). For each group and condition, eight adults were collected and adapted with food in complete dark for >2 h. After exposure for 60 s to pure blue light (480 ± 10 nm), fly heads were isolated by liquid nitrogen and homogenized in the dark. After centrifuging at 14,600 × g for 5 min, pellet and supernatant fractions were separated under very dim red light for Western blotting analysis. Arr2 release assays were performed in the same manner, except that the flies were exposed to 60 s of pure blue light followed by 2 min of pure orange light (580 ± 10 nm).
Statistics
For each quantitative analysis, the images of Western blots and immunostaining were quantified using ImageJ software. After averaging three sets of data, the mean values ± S.E. (as error bars) are presented.
RESULTS
Isolation and Identification of a novel Gαq Mutant Allele
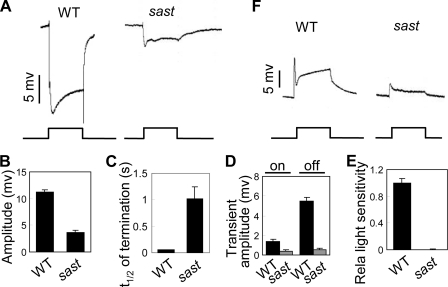
To characterize the components of the phototransduction machinery, we performed an ERG-based chemical mutagenesis screen for additional genes in the fly. We isolated a mutant fly, sast (small amplify and slow termination), which shows small ERG responses (3.7 ± 0.3 mV versus 11.2 ± 0.4 mV) and slow photoresponse termination (t½ = 1.02 ± 0.22 s versus t½ = 0.06 ± 0.01 s) compared with wild-type flies (Fig. 1, A–C). In addition, sast mutant displays the reduced ON transient and OFF transient (Fig. 1, A and D). The light sensitivity of this mutant is also significantly reduced (Fig. 1E). Intracellular recording further revealed that the defective light response in sast mutants was due to an abnormality in photoreceptor cells (Fig. 1F).
FIGURE 1.
sast mutants show abnormal light responses. A, representative ERG traces of wild-type and sast mutant flies. For all ERG traces, event markers represent 5-s orange light pulses, and scale bars are 5 mV. B, quantification of ERG amplitudes for each genotype. C, quantification of termination speed. t½ was calculated as described under “Experimental Procedures.” Error bars indicate S.E. D, quantification of ON/OFF transient amplitudes. For each genotype, 10 flies were examined, and the ON/OFF transient amplitudes were averaged to obtain the mean. E, quantification of relative light sensitivities. The shown mean relative sensitivities were calculated as described under “Experimental Procedures.” Error bars indicate S.E. F, intracellular recordings of light responses in photoreceptors. The scale bar and light pulse are 5 mV and 5 s, respectively.
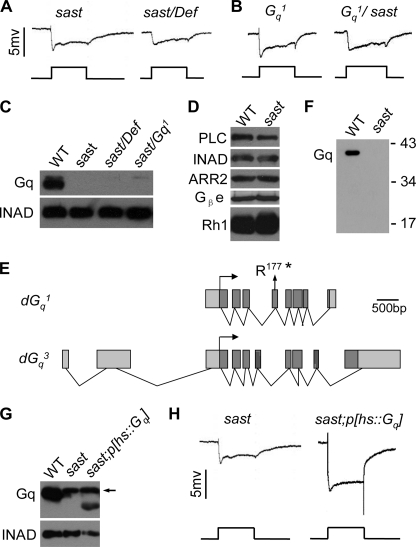
To identify the specific mutation in sast flies, we first mapped the mutation to the 49B5–49B12 chromosomal region based on the ERG phenotype covered by the deficient chromosome Df(2R)Exel7121 (missing 49B5–10 to 49B12) (Fig. 2A). This genomic region contains 26 genes, including Gα49B, which encodes the Gq protein. Because the mutant flies showed an ERG phenotype similar to that of the Gαq1 mutants, the sast mutant might harbor a Gαq mutant allele. The sast/Gαq1 flies displayed an ERG phenotype similar to that of either the sast or Gαq1 mutant (Fig. 2B), supporting this conclusion.
FIGURE 2.
sast is a novel Gαq mutant allele. A, sast over Df(2R)Exel7121 heterozygote (sast/Def) showing ERG responses similar to those of the sast homozygote. Event markers represent 5-s orange light pulses. B, sast over Gαq1, heterozygote (Gαq1/sast) showing ERG responses similar to those of the sast homozygote. C, Western blots of DGq protein levels in the sast mutant. Each lane was loaded with one-eighth head. Note that the upper weak bands are nonspecific. D, Western blots of protein levels of other visual molecules in the sast mutant. Each lane was loaded with one-quarter head. E, annotated splice variants of the Gα49B gene. The position of the point mutation in the sast allele is shown. The translation starts in each of the splice variants are marked with arrows. *, stop codon. F, Western blots of truncated DGq protein in the sast mutants. Each lane was loaded with three isolated retinas and detected with an antibody that specifically recognizes the N terminus of DGq. G, DGq protein levels recovered in sast;p[hs:: Gαq] flies. The flies were heat shocked at the late pupal stage by immersing fly vials in a 37 °C water bath for 1 h every day. Note that the upper band pointed with arrow is a nonspecific band. H, ERG recordings of light responses in sast;p[hs:: dGαq] flies. Flies were treated as described in G.
A previous study showed that the Gα49B gene encodes several DGq splice variants (27) and that DGq1 plays a predominant role in phototransduction in Drosophila (4). Using an antibody that recognizes DGq1, we revealed that the DGq1 protein is absent in sast mutants (Fig. 2C and supplemental Fig. 1). In contrast, other phototransduction proteins were present at normal levels (Fig. 2D). Subsequent DNA sequencing revealed that the sast mutant contains a C961T mutation in the Gα49B gene (Fig. 2E). This mutation corresponds to a nonsense mutation (Arg117 to stop codon) in exon 4 (amino acids 153–196 of DGq1; Fig. 2E), which is not included in the DGq3 and DGq4 variants (27). To examine whether the sast mutant retains any truncated DGq1 protein, we performed Western blotting with an antibody against the N terminus of DGq1. We could not detect any truncated DGq1 protein, suggesting that the sast mutant is likely to possess one Gαq1-null allele (Fig. 2F). Therefore, we named the sast mutant Gαq961.
Because Gαq961 is a Gαq1-null mutant and DGq1 plays a predominant role in phototransduction in Drosophila, we worried whether the residual light response observed in the Gαq961 mutant was evoked by either DGq3 or DGq4 variants. To detect DGq3 and DGq4 protein in both Gq1 and Gαq961 mutants, we performed Western blotting by using the anti-Gq α subunit antibody (371754; Calbiochem) that also recognizes DGq3 and DGq4 variants. Unfortunately, we did not detect any DGq3 or DGq4 in either Gq1 and Gαq961 mutants even loading with 32 isolated retinas (data not shown). This observation implied that the expressions of DGq3 or DGq4 in the retina were extremely low and the residual light response in the Gαq961 mutant might be attributable to other G protein genes, but not the Gq49B gene. To further support this hypothesis, we generated clones of Gα49B-nulls in the retina through ey-FLP-induced FRT recombination in deficiency line, Df(2R)Exel7121, which deletes the whole Gα49B gene. This fly also showed small light response and slow termination ERG phenotype (supplemental Fig. 2). This observation indicates that the residual light response observed in the Gαq961 mutant is contributed by other G protein, but not DGq3 or DGq4.
To further confirm that the absence of the DGq1 protein is indeed responsible for the Gαq961 mutant phenotype, we performed rescue experiments by expressing DGq1 protein in a Gαq961 mutant background. After induction of heat shock, the levels of the DGq1 protein were recovered (Fig. 2G), and ERG responses were restored in Gαq961;p[hs-dGαq] flies (Fig. 2H). These results illuminate that the loss of DGq1 leads to light response defects in Gαq961 flies.
DGq Is Essential for Deactivation of Photoresponse
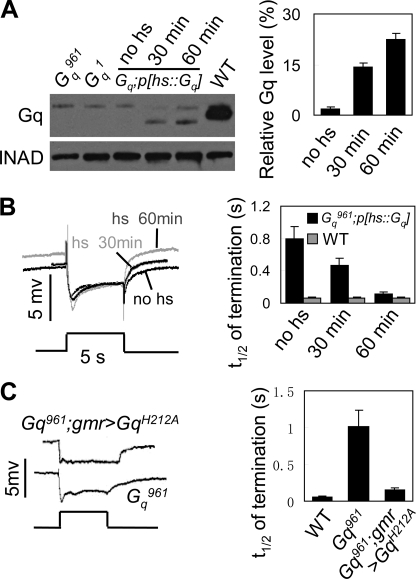
In the rescue experiments, we observed that the deactivation kinetics of the photoresponse correlated with the available amount of DGq protein. In Gαq961;p[hs-dGαq] flies, in vivo DGq protein levels could be manipulated by controlling the time of heat shock. Within 30 min of heat shock at 37 °C, DGq protein levels were partial recovered (14.2 ± 1.3%, Fig. 3A), accompanied by an increase in the termination speed (t½ = 0.47 ± 0.08, Fig. 3B). Extended heat shock induced further increases in the levels of DGq protein (22.4 ± 1.7%, Fig. 3A) and triggered rapid termination (t½ = 0.11 ± 0.01, Fig. 3B). We excluded the possibility that the increased termination speed was due to the heat-shock treatment, because the heat-shock treatment did not change the protein level of DGq and the termination speed in wild-type flies (Fig. 3B, right, and supplemental Fig. 3). These data suggest that termination speed depends on the available amount of the DGq protein.
FIGURE 3.
DGq is required for termination of light response. A, Gq protein levels in different treatments. cn,Gαq961,bw; P[hs::dGαq] adults reared at 21 °C were exposed to 30 min of heat shock at 37 °C (30 min) or 1 h of heat shock at 37 °C (60 min) or no heat shock (no hs). All flies were collected and analyzed at 24 h after heat shock. INAD was used as the loading control. Note that the upper bands are nonspecific bands. Quantification of relative DGq protein levels is shown on the right. B, ERG recordings showing termination kinetics of light responses in each treatment. Gq protein was induced as described in A. For all ERG traces, event markers represent 5-s orange light pulses, and the treatment conditions for each trace are marked. Quantification of the time required for half-recovery in each treatment, including wild-type controls, is shown on the right. C, representative ERG traces of cn,Gαq961,bw and cn,Gαq961,bw;gmr>GqH212A flies. Quantification of the time required for half-recovery in each treatment is shown on the right. Error bars indicate S.E.
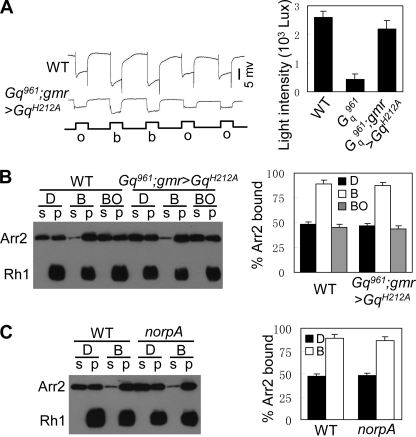
Previous work has shown that Gαq1 mutants generate quantum bumps with prolonged latency (4). It is possible that the slow termination phenotype reflects a slow activation process. To test this hypothesis, we generated a variant DGqH212A transgenic fly with a mutated His212 to Ala, resulting in a loss of capacity of Gq to activate PLC (28). In cn,Gαq961,bw; gmr>DGqH212A flies, the levels of variant DGqH212A protein were comparable with the levels of Gq protein in wild-type flies (supplemental Fig. 4). Interestingly, deactivation kinetics, but not ERG amplitude, was nearly restored in this fly (Fig. 3C). These results indicate that the slow termination phenotype in Gαq mutants is not due to delayed bump generation.
DGq Mediates Deactivation of Metarhodopsin
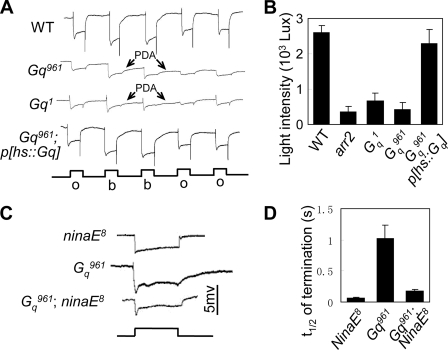
During fly photoresponse termination, metarhodopsin deactivation is the most important step (29). Thus, we first investigated whether DGq was required for the deactivation of metarhodopsin. Prolonged depolarization afterpotential (PDA) is induced when the number of activated rhodopsin molecules exceeds the number of available regulators (29). In mutants lacking rhodopsin regulators, because lower amounts of rhodopsin need to be activated, the light intensity required to induce PDA is much lower than that in wild-type flies (14). The minimum light intensity needed to induce PDA was approximately 420 lux in Gαq961 versus approximately 2,600 lux in wild-type and 2300 lux in cn,Gαq961,bw;P[hs::Gαq] flies (Fig. 4, A and B). Therefore, this result suggests that the deactivation of metarhodopsin is impaired and that there is a shortage of rhodopsin regulator molecules in Gαq961 mutant.
FIGURE 4.
DGq mediates deactivation of metarhodopsin. A, representative recorded traces of PDA induced by 600 lux (low intensity) blue light. All flies were in white-eye background and reared in the dark. o, orange light; b, blue light. B, quantification of minimum light intensities required for PDA production in each genotype. C, representative ERG traces of cn,Gαq961,bw and cn,Gαq961,bw;ninaE8 double mutant flies. D, quantification of the time required for half-recovery in each genotype. Error bars, S.E.
Because the deactivation of metarhodopsin is impaired in Gαq961 mutants, we attempted to increase the termination speed of Gαq961 photoresponses by reducing the level of Rh1, the major rhodopsin in all outer (R1–R6) photoreceptor cells. We genetically reduced Rh1 levels via the introduction of ninaE8, which contains <1% Rh1 protein (30), into a Gαq961 background. Because much less Rh1 need to be deactivated, the impaired regulating machinery might be sufficient for the deactivation of rhodopsin, and the termination speed of the photoresponse should be faster in Gαq961;ninaE8 double mutants compared with that in the Gαq961 single mutant. Indeed, in Gαq961;ninaE8 double mutants, the deactivation of the photoresponse was much faster than that in the Gαq961 single mutant (Fig. 4, C and D), suggesting that DGq is required for the deactivation of metarhodopsin.
Metarhodopsin/Gq Interaction Affects Arr2-Rh1 Binding
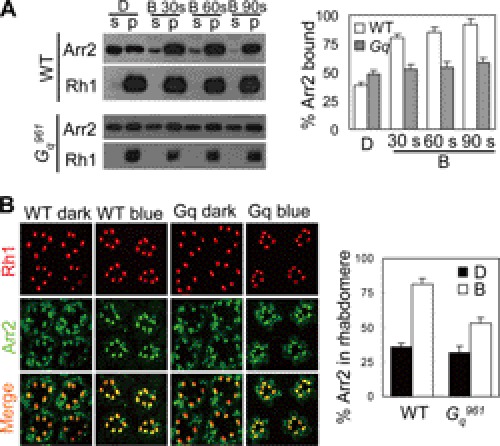
Because Arr2 is the primary deactivator of rhodopsin through its binding with rhodopsin (11, 12), we next assessed whether Gq mediates the deactivation of metarhodopsin by affecting Arr2-Rh1 binding. To characterize the Arr2/Rh1 interaction, we used an arrestin pelleting assay (31, 32). Upon 30 s of blue light stimulation, >80% Arr2 binds to metarhodopsin in wild-type flies, whereas only 52% Arr2 binds to metarhodopsin in the Gαq961 mutant (80.1 ± 3.2% versus 52.3 ± 4.3%, Fig. 5A, middle panel). Immunostaining results were consistent with those of the arrestin pelleting assay (Fig. 5B). These observations suggest that DGq indeed affects Arr2-Rh1 binding.
FIGURE 5.
Lack of Gq leads to the impaired Arr2-Rh1 binding. A, assessment of deficits in Arr2-Rh1 binding. Dark-adapted flies were exposed to blue light for 30, 60, or 90 s, respectively. Supernatant (S) and membrane pellet (P) fractions of fly heads were subjected to Western blotting. D, kept in dark; B, blue light exposure. Quantification of Arr2-Rh1 binding is shown on the right. B, immunostaining of Arr2 and Rh1 in different genotypes and under different conditions. Sections were prepared as described under “Experimental Procedures.” Quantification of Arr2 in rhabdomeres is shown on the right. The averaged data from three independent sections are shown. Error bars, S.E.
Because Gq functions as a molecular switch on phototransduction cascade, we next investigated whether Gq affects Arr2-Rh1 binding by activating the phototransduction cascades. In cn,Gαq961,bw;grm>GqH212A flies, phototransduction cascade cannot be activated by the variant DGqH212A (Fig. 3C). Interestingly, the minimum light intensity needed to induce PDA in this fly (2200 ± 300 lux) was comparable with that in wild-type flies (2600 ± 200 lux) (Fig. 6A), reflecting the normal Arr2-Rh1 binding. An Arr2-binding assay further showed that Arr2-Rh1 binding is normal in these flies (Fig. 6B). Furthermore, Arr2-Rh1 binding is normal in norpA mutants, which also show defects in the activation of phototransduction (Fig. 6C) (33). Taken together, these observations suggest that the metarhodopsin/Gq interaction, but not the activation of the phototransduction cascade, affects Arr2-Rh1 binding.
FIGURE 6.
Metarhodopsin/Gq interactions affect Arr2-Rh1 binding. A, minimum light intensities required for PDA production in cn,Gαq961,bw;gmr>GqH212A flies, comparable with that in wild-type flies. Representative recorded traces were induced by 2000 lux blue light. All flies were dark-reared. o, orange light; b, blue light. Quantification of minimum light intensities required for PDA production in each genotype is shown on the right. B, Arr2-Rh1 binding in cn,Gαq961,bw;gmr>GqH212A flies. Arr2-Rh1 binding and Arr2 release assays were performed as described under “Experimental Procedures.” Quantification of relative Arr2-Rh1 binding and release is shown on the right. C, Arr2-Rh1 binding in norpA mutants. Arr2-Rh1 binding assays were performed as described under “Experimental Procedures.” Quantification of the relative Arr2-Rh1 binding is shown on the right. Error bars, S.E.
Gαq961 Mutant Undergoes Light-dependent Retinal Degeneration
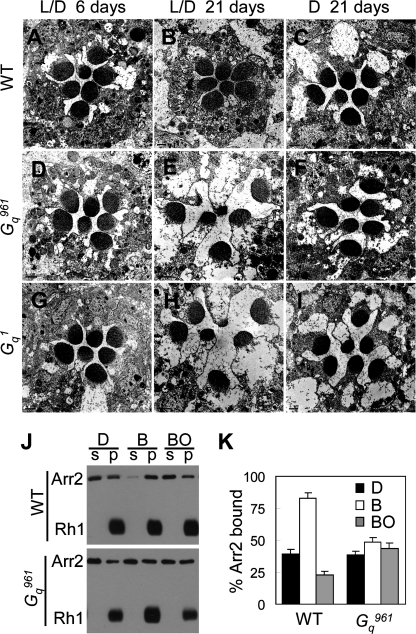
In Drosophila, mutations in almost any gene involved in the phototransduction cascade lead to light-dependent retinal degeneration (34). Thus, we investigated whether the Gαq961 mutant and Gαq1 mutant also undergo retinal degeneration. An EM study showed obvious retinal degeneration in older mutants but not in 6-day-old mutants (Fig. 7, A, B, D, E, G, and H). Additionally, 21-day-old dark-reared mutants did not undergo retinal degeneration (Fig. 7, C, F, and I). These observations indicate that Gαq961 mutants and the Gαq1 mutant undergo slow light-dependent retinal degeneration.
FIGURE 7.
Gαq961 mutants undergo slow light-dependent retinal degeneration. EM sections were prepared to assess retinal degeneration as described under “Experimental Procedures.” Wild type (A–C), cn,Gαq961,bw (D–F), and Gq1 (G–I) flies were raised for 6 (A, D, and G) or 21 days (B, C, E, F, H, and I). L/D flies (A, B, D, E, G, and H) were reared under 12-h light/12-h dark conditions, and D flies (C, F, and I) were kept in constant darkness. Scale bars of EM micrographs are 1 μm. J, Western blot of fractions of wild-type and cn,Gαq961,bw fly heads. Arr2-Rh1 binding assays were performed as described under “Experimental Procedures.” K, quantification of the percentage of Arr2 bound to rhodopsin-containing membranes in the dark (D), after treatment with blue light (B), or after treatment with blue light followed by orange light (BO). Error bars, S.E.
PLCβ, encoded by norpA, is the downstream effector of Gq (33). A loss of PLC results in the formation of stable Arr2-Rh1 complexes, which trigger retinal degeneration (32). Hence, we examined whether the same mechanism causes retinal degeneration in the Gαq961 mutant. With 480-nm blue light exposure, Rh1 is photoconverted to metarhodopsin and induces binding with Arr2. Metarhodopsin can be photoconverted to inactivated rhodopsin, leading to the release of Arr2 (32). In wild-type flies, blue light exposure caused >80% binding between Arr2 and Rh1 and >80% release of Arr2 from Rh1 following exposure to orange light (Fig. 7, J and K). In contrast, in the mutant, blue light exposure only triggered approximate 48.5% binding between Arr2 and Rh1, and <60% release of Arr2 from Rh1 following exposure to orange light (Fig. 7, J and K). These data imply that the formation of stable Arr2-Rh1 complexes might trigger retinal degeneration in the Gαq mutant.
DISCUSSION
Our work isolated and identified a novel Gαq mutant allele and demonstrated that metarhodopsin/Gq interaction affects subsequent Arr2-Rh1 binding, which might mediate the deactivation of metarhodopsin. Our study revealed the involvement of a general rapid feedback regulation in GPCR signaling.
Gq Mediates Termination of Light Response
Rapid termination of the light response is critical for fly vision to achieve high temporal resolution and to prevent excessive calcium-induced cell toxicity (9, 10). Using an ERG-based chemical mutagenesis screen, we isolated a novel Gαq mutant allele, Gαq961, which shows defects in the termination of the light response. A similar phenotype has been reported in another Gαq mutant allele, Gαq1 (4). Compared with the Gαq1 mutant, Gαq961 showed even more severe defects in photoresponse deactivation. One explanation is that Gαq961 mutants contain an even lower amount of Gq protein than that found in Gαq1 mutants. Western blotting revealed that the Gαq961 mutant is likely a null mutant. In contrast, Gαq1 mutants produce approximately 1% of wild-type levels of the DGq protein (4). This explanation is further supported by rescue experiments, which showed that the termination kinetics depend on the amount of available Gq protein.
A previous study showed that the absence of Gq leads to the generation of quantum bumps with prolonged latency (4) because it takes longer for rare residual Gq to encounter metarhodopsin. In this study, we provided evidence that the slow termination phenotype of the Gαq mutants was not due to slow activation. Expression of DGqH212A, which can bind metarhodopsin but cannot activate PLC, is nearly able to restore the defects of photoresponse termination in a Gαq961 mutant background. In Gαq961;gmr>GqH212A flies, the termination speed of the photoresponse is slower than that in wild-type flies, which might be attributed to slow activation and impaired calcium-dependent receptor deactivation mechanisms (35).
Metarhodopsin/Gq Interaction Affects Arr2-Rh1 Binding
Combined with genetic, biochemical, and electrophysiological approaches, we showed that metarhodopsin/Gq interaction affects subsequent Arr2-Rh1 binding. Using the arrestin pelleting assay, we showed that more Arr2 binds to metarhodopsin in wild-type flies than that in the Gαq961 mutant upon blue light stimulation (80.1 ± 3.2% versus 52.3 ± 4.3%). Although some of the Arr2 can still bind with Rh1 in the Gαq961 mutant, the metarhodopsin/Gq interaction clearly affects subsequent Arr2-Rh1 binding, which mediates the deactivation of photoresponse. This conclusion is further supported by the observations in cn,Gαq961,bw;grm>GqH212A flies. We showed the rapid deactivation kinetics of ERG, normal Arr2-Rh1 binding, and comparable minimum light intensity needed to induce PDA in this fly. This rapid feedback regulation does not need to activate the entire phototransduction cascade and allows the termination of photoreceptors with extremely fast kinetics, permitting adaptation in a huge dynamic range of light intensities (10, 34, 36). Such regulation also prevents excessive Ca2+ influx and Ca2+-dependent excitotoxicity during intensive stimulation (37–40).
It is possible that the metarhodopsin/Gq interaction exposes the binding site in metarhodopsin or shifts metarhodopsin into an intermediate metarhodopsin status, which facilitates Arr2-Rh1 binding. Using fluorescence pump probe and time-resolved fluorescence depolarization measurement approaches, it has been shown that helix 8 of rhodopsin undergoes dynamic changes at the receptor surface, which modulates arrestin-rhodopsin binding (41). In vertebrates, the phosphorylation of rhodopsin is important for β-arrestin/rhodopsin binding. GPCR kinase 2 has been shown to undergo selective binding with the Gq α subunit (42). These results indicate that the rhodopsin/Gq interaction might mediate arrestin-rhodopsin binding via the promotion of rhodopsin phosphorylation. However, this is not the case in the deactivation of the photoresponse in the fly because the phosphorylation of metarhodopsin is not required for Arr2-Rh1 binding in fly photoreceptors (14, 32, 43).
Gαq Mutant Undergoes Slow Retinal Degeneration
Mutations in almost any gene that functions during phototransduction trigger rapid retinal degeneration, except the Gαq1 mutant, which undergoes slow retinal degeneration (34, 43, 44). In this study, we showed that the Gαq961 mutant undergoes slow light-dependent retinal degeneration, similar to that observed in the Gαq1 mutant (44). We provided evidence that mild retinal degeneration in the Gαq961 mutant was due to the slow accumulation of stable Arr2-Rh1 complexes, which trigger retinal degeneration (32). Light stimulation triggers Ca2+ influx and activates CaM kinase II, which phosphorylates Arr2 and results in Arr2 release from Rh1 (26, 45). In the Gαq961 mutant, impaired Ca2+ influx leads to the slow release of Arr2 from Rh1. On the other hand, lack of Gq partially inhibits basal Rh1 endocytosis (24) and inhibits the translocation of Arr2 to rhabdomeres (46). These two opposite effects lead to the slow accumulation of stable Arr2-Rh1 complexes and trigger mild retinal degeneration.
Supplementary Material
Acknowledgments
We thank Charles S. Zuker for Gαq1 mutant flies and p[hs::dGαq] transgenic flies, Craig Montell for anti-INAD antibody, Reinhard Paulsen for anti-Gβe antibody, the Bloomington Stock Center for the flies; and people in the Han laboratory for critical comments on the manuscript.
This work was supported by National Natural Science Foundation of China Grants 30970663 and 31070683 (to J. H.).

This article contains supplemental Figs. 1–4.
- PLC
- phospholipase C
- Arr2
- arrestin
- ERG
- electroretinogram
- GPCR
- G protein-coupled receptor
- INAD
- inactivation no afterpotential D
- PDA
- depolarization afterpotential
- Rh1
- rhodopsin.
REFERENCES
- 1. Pierce K. L., Premont R. T., Lefkowitz R. J. (2002) Seven-transmembrane receptors. Nat. Rev. Mol. Cell. Biol. 3, 639–650 [DOI] [PubMed] [Google Scholar]
- 2. Neer E. J. (1995) Heterotrimeric G proteins: organizers of transmembrane signals. Cell 80, 249–257 [DOI] [PubMed] [Google Scholar]
- 3. Lee Y. J., Shah S., Suzuki E., Zars T., O'Day P. M., Hyde D. R. (1994) The Drosophila dgq gene encodes a Gα protein that mediates phototransduction. Neuron 13, 1143–1157 [DOI] [PubMed] [Google Scholar]
- 4. Scott K., Becker A., Sun Y., Hardy R., Zuker C. (1995) Gqα protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron 15, 919–927 [DOI] [PubMed] [Google Scholar]
- 5. Montell C., Rubin G. M. (1989) Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2, 1313–1323 [DOI] [PubMed] [Google Scholar]
- 6. Hardie R. C., Minke B. (1992) The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 8, 643–651 [DOI] [PubMed] [Google Scholar]
- 7. Phillips A. M., Bull A., Kelly L. E. (1992) Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron 8, 631–642 [DOI] [PubMed] [Google Scholar]
- 8. Ranganathan R., Harris G. L., Stevens C. F., Zuker C. S. (1991) A Drosophila mutant defective in extracellular calcium-dependent photoreceptor deactivation and rapid desensitization. Nature 354, 230–232 [DOI] [PubMed] [Google Scholar]
- 9. Montell C. (1999) Visual transduction in Drosophila. Annu. Rev. Cell Dev. Biol. 15, 231–268 [DOI] [PubMed] [Google Scholar]
- 10. Hardie R. C., Raghu P. (2001) Visual transduction in Drosophila. Nature 413, 186–193 [DOI] [PubMed] [Google Scholar]
- 11. Satoh A. K., Ready D. F. (2005) Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr. Biol. 15, 1722–1733 [DOI] [PubMed] [Google Scholar]
- 12. Ranganathan R., Stevens C. F. (1995) Arrestin binding determines the rate of inactivation of the G protein-coupled receptor rhodopsin in vivo. Cell 81, 841–848 [DOI] [PubMed] [Google Scholar]
- 13. Scott K., Zuker C. (1997) Lights out: deactivation of the phototransduction cascade. Trends Biochem. Sci. 22, 350–354 [DOI] [PubMed] [Google Scholar]
- 14. Vinós J., Jalink K., Hardy R. W., Britt S. G., Zuker C. S. (1997) A G protein-coupled receptor phosphatase required for rhodopsin function. Science 277, 687–690 [DOI] [PubMed] [Google Scholar]
- 15. Ranganathan R., Bacskai B. J., Tsien R. Y., Zuker C. S. (1994) Cytosolic calcium transients: spatial localization and role in Drosophila photoreceptor cell function. Neuron 13, 837–848 [DOI] [PubMed] [Google Scholar]
- 16. Hardie R. C., Minke B. (1994) Calcium-dependent inactivation of light-sensitive channels in Drosophila photoreceptors. J. Gen. Physiol. 103, 409–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scott K., Sun Y., Beckingham K., Zuker C. S. (1997) Calmodulin regulation of Drosophila light-activated channels and receptor function mediates termination of the light response in vivo. Cell 91, 375–383 [DOI] [PubMed] [Google Scholar]
- 18. Smith D. P., Ranganathan R., Hardy R. W., Marx J., Tsuchida T., Zuker C. S. (1991) Photoreceptor deactivation and retinal degeneration mediated by a photoreceptor-specific protein kinase C. Science 254, 1478–1484 [DOI] [PubMed] [Google Scholar]
- 19. Porter J. A., Yu M., Doberstein S. K., Pollard T. D., Montell C. (1993) Dependence of calmodulin localization in the retina on the NINAC unconventional myosin. Science 262, 1038–1042 [DOI] [PubMed] [Google Scholar]
- 20. Porter J. A., Minke B., Montell C. (1995) Calmodulin binding to Drosophila NinaC required for termination of phototransduction. EMBO J. 14, 4450–4459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stowers R. S., Schwarz T. L. (1999) A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics 152, 1631–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han J., Gong P., Reddig K., Mitra M., Guo P., Li H. S. (2006) The fly CAMTA transcription factor potentiates deactivation of rhodopsin, a G protein-coupled light receptor. Cell 127, 847–858 [DOI] [PubMed] [Google Scholar]
- 23. Cao J., Li Y., Xia W., Reddig K., Hu W., Xie W., Li H. S., Han J. (2011) A Drosophila metallophosphoesterase mediates deglycosylation of rhodopsin. EMBO J. 30, 3701–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han J., Reddig K., Li H. S. (2007) Prolonged Gq activity triggers fly rhodopsin endocytosis and degradation, and reduces photoreceptor sensitivity. EMBO J. 26, 4966–4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schulz S., Huber A., Schwab K., Paulsen R. (1999) A novel Gγ isolated from Drosophila constitutes a visual G protein γ subunit of the fly compound eye. J. Biol. Chem. 274, 37605–37610 [DOI] [PubMed] [Google Scholar]
- 26. Alloway P. G., Dolph P. J. (1999) A role for the light-dependent phosphorylation of visual arrestin. Proc. Natl. Acad. Sci. U.S.A. 96, 6072–6077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ratnaparkhi A., Banerjee S., Hasan G. (2002) Altered levels of Gq activity modulate axonal pathfinding in Drosophila. J. Neurosci. 22, 4499–4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Waldo G. L., Ricks T. K., Hicks S. N., Cheever M. L., Kawano T., Tsuboi K., Wang X., Montell C., Kozasa T., Sondek J., Harden T. K. (2010) Kinetic scaffolding mediated by a phospholipase Cβ and Gq signaling complex. Science 330, 974–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dolph P. J., Ranganathan R., Colley N. J., Hardy R. W., Socolich M., Zuker C. S. (1993) Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science 260, 1910–1916 [DOI] [PubMed] [Google Scholar]
- 30. Scavarda N. J., O'tousa J., Pak W. L. (1983) Drosophila locus with gene-dosage effects on rhodopsin. Proc. Natl. Acad. Sci. U.S.A. 80, 4441–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kiselev A., Subramaniam S. (1997) Studies of Rh1 metarhodopsin stabilization in wild-type Drosophila and in mutants lacking one or both arrestins. Biochemistry 36, 2188–2196 [DOI] [PubMed] [Google Scholar]
- 32. Alloway P. G., Howard L., Dolph P. J. (2000) The formation of stable rhodopsin-arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron 28, 129–138 [DOI] [PubMed] [Google Scholar]
- 33. Bloomquist B. T., Shortridge R. D., Schneuwly S., Perdew M., Montell C., Steller H., Rubin G., Pak W. L. (1988) Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell 54, 723–733 [DOI] [PubMed] [Google Scholar]
- 34. Wang T., Montell C. (2007) Phototransduction and retinal degeneration in Drosophila. Pflugers Arch. 454, 821–847 [DOI] [PubMed] [Google Scholar]
- 35. Liu C. H., Satoh A. K., Postma M., Huang J., Ready D. F., Hardie R. C. (2008) Ca2+-dependent metarhodopsin inactivation mediated by calmodulin and NINAC myosin III. Neuron 59, 778–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Juusola M., Hardie R. C. (2001) Light adaptation in Drosophila photoreceptors: I. Response dynamics and signaling efficiency at 25 ºC. J. Gen. Physiol. 117, 3–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Orrenius S., Zhivotovsky B., Nicotera P. (2003) Regulation of cell death: the calcium-apoptosis link. Nat. Rev. 4, 552–565 [DOI] [PubMed] [Google Scholar]
- 38. Hotta Y., Benzer S. (1970) Genetic dissection of the Drosophila nervous system by means of mosaics. Proc. Natl. Acad. Sci. U.S.A. 67, 1156–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raghu P., Usher K., Jonas S., Chyb S., Polyanovsky A., Hardie R. C. (2000) Constitutive activity of the light-sensitive channels TRP and TRPL in the Drosophila diacylglycerol kinase mutant, rdgA. Neuron 26, 169–179 [DOI] [PubMed] [Google Scholar]
- 40. Porter J. A., Hicks J. L., Williams D. S., Montell C. (1992) Differential localizations of and requirements for the two Drosophila ninaC kinase/myosins in photoreceptor cells. J. Cell Biol. 116, 683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kirchberg K., Kim T. Y., Möller M., Skegro D., Dasara Raju G., Granzin J., Büldt G., Schlesinger R., Alexiev U. (2011) Conformational dynamics of helix 8 in the GPCR rhodopsin controls arrestin activation in the desensitization process. Proc. Natl. Acad. Sci. U.S.A. 108, 18690–18695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carman C. V., Parent J. L., Day P. W., Pronin A. N., Sternweis P. M., Wedegaertner P. B., Gilman A. G., Benovic J. L., Kozasa T. (1999) Selective regulation of Gαq/11 by an RGS domain in the G protein-coupled receptor kinase, GRK2. J. Biol. Chem. 274, 34483–34492 [DOI] [PubMed] [Google Scholar]
- 43. Kiselev A., Socolich M., Vinós J., Hardy R. W., Zuker C. S., Ranganathan R. (2000) A molecular pathway for light-dependent photoreceptor apoptosis in Drosophila. Neuron 28, 139–152 [DOI] [PubMed] [Google Scholar]
- 44. Iakhine R., Chorna-Ornan I., Zars T., Elia N., Cheng Y., Selinger Z., Minke B., Hyde D. R. (2004) Novel dominant rhodopsin mutation triggers two mechanisms of retinal degeneration and photoreceptor desensitization. J. Neurosci. 24, 2516–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matsumoto H., Kurien B. T., Takagi Y., Kahn E. S., Kinumi T., Komori N., Yamada T., Hayashi F., Isono K., Pak W. L. (1994) Phosrestin I undergoes the earliest light-induced phosphorylation by a calcium/calmodulin-dependent protein kinase in Drosophila photoreceptors. Neuron 12, 997–1010 [DOI] [PubMed] [Google Scholar]
- 46. Satoh A. K., Xia H., Yan L., Liu C. H., Hardie R. C., Ready D. F. (2010) Arrestin translocation is stoichiometric to rhodopsin isomerization and accelerated by phototransduction in Drosophila photoreceptors. Neuron 67, 997–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.