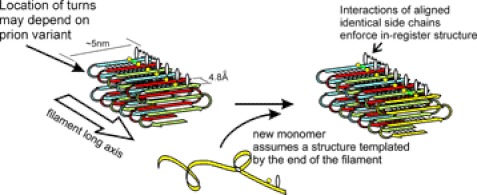
FIGURE 3.
The in-register parallel β-sheet architecture with longitudinal folds of yeast prion amyloid filaments can explain transmission of conformational information to monomers joining the end of the filament. The same interactions among aligned identical amino acid side chains that hold the structure in-register also drive the monomer joining the end of the filament to have the same conformation as the molecules already in the filament (103, 106).