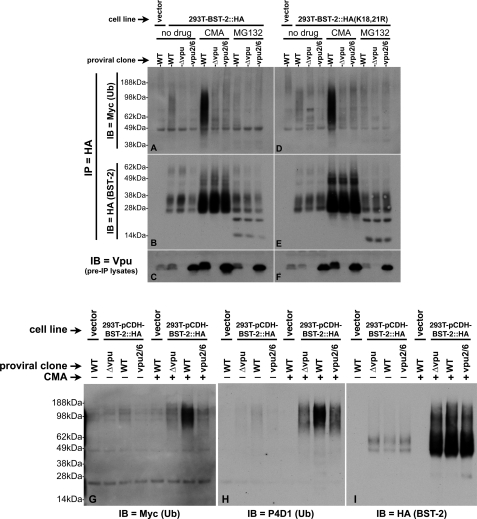
FIGURE 3.
Comparison of the ubiquitination status of WT BST-2 and BST-2(K18R,K21R). A, 293T::BST-2::HA cells that had been transfected with pMyc::Ub and the indicated pNL4–3-based proviral clones were treated with either CMA, MG132, or carrier (DMSO). A 293T cell line transduced with the empty lentiviral vector, which does not express BST-2, was transfected with both pMyc::Ub and pNL4–3 and then treated with carrier only (left lane). 100 μg each of lysates made from these cells were immunoprecipitated with an HA mAb directly conjugated to agarose beads. Shown here is an immunoblot (IB) of the IPs probed with an anti-Myc antibody to detect ubiquitin conjugated to BST-2::HA. B, the immunoblot shown in A was stripped and reprobed with an anti-BST-2 polyclonal antisera. C, an immunoblot of the input lysates used in A and B probed with an anti-Vpu polyclonal antibody shows the level of Vpu expressed in these cells. D–F, 293T::BST-2::HA(K18R,K21R) cells were subjected to the identical treatment and analyses described for the 293T::BST-2::HA cells in A–C. G, 20 μg each of a subset of the samples described in A were once again subjected to IP with HA-agarose beads followed by SDS-PAGE and immunoblotting with an anti-Myc antibody (left panel). The same blot was then sequentially stripped and reprobed, first with the anti-ubiquitin antibody P4D1 (H) and finally an HA antibody (I) to demonstrate levels of BST-2 recovered in each IP.