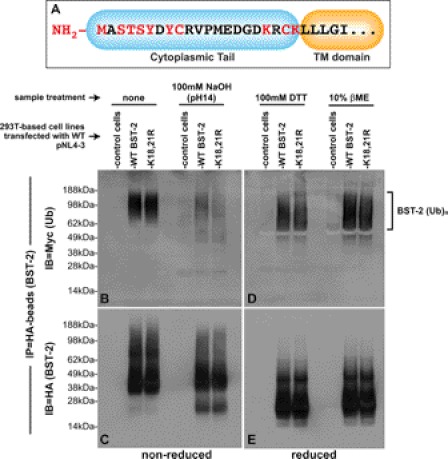
FIGURE 4.
Chemical cleavage of ubiquitin moieties from BST-2::HA: Vpu promotes the ubiquitination of BST-2 on serine/threonine/tyrosine. A, shown is a schematic of the BST-2 cytoplasmic domain, with potential ubiquitin target residues highlighted in red. B, immunoprecipitated samples of ubiquitinated BST-2::HA and BST-2::HA(K18R,K21R) were generated as described in Fig. 3, including a sample prepared from control cells that do not express BST-2::HA. Those samples were then left untreated (left three lanes) or were treated with 100 mm NaOH. The proteins were then separated via non-reducing SDS-PAGE. An immunoblot (IB) of the gel was then probed with an anti-Myc mAb to detect ubiquitinated BST-2::HA species. C, the immunoblot in B was stripped and reprobed with an anti-HA mAb to detect BST-2::HA. D, immunoprecipitated samples of ubiquitinated BST-2::HA and BST-2::HA(K18R,K21R) were generated as described in Fig. 3, including a sample prepared from control cells that do not express BST-2::HA. The immunoprecipitates were resuspended in sample buffer supplemented with either 100 mm DTT (first three lanes) or 10% βME (last three lanes). The proteins were then separated via reducing SDS-PAGE (+DTT). An immunoblot of the gel was probed with an anti-Myc mAb. E, the immunoblot in D was stripped and reprobed with an anti-HA mAb to detect BST-2::HA.