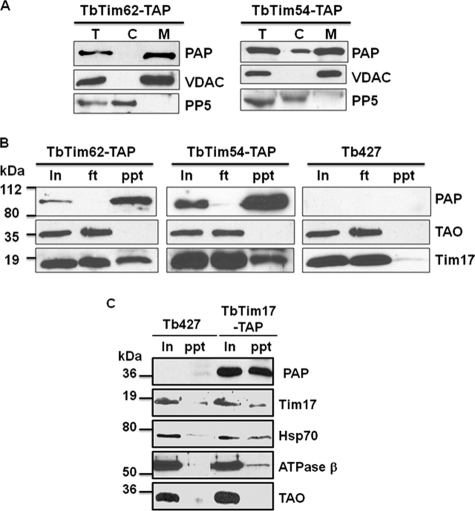
FIGURE 9.
Co-precipitation of TbTim17 with TbTim54, TbTim62, and Hsp70. A, expression of the C-terminal TAP-tagged TbTim62 and TbTim54. Transfected cell lines containing pLew79-MHT/TbTim62 (TbTim62-TAP) or pLew79-MHT/TbTim54 (TbTim54-TAP) were induced separately with doxycycline for 48 h. Cells were harvested, and subcellular fractions were prepared as described under “Experimental Procedures.” The total (T), cytosolic (C), and mitochondrial (M) fractions were analyzed by SDS-PAGE and immunoblot using PAP reagent for detection of the protein A tag. VDAC and PP5 were used as the markers for mitochondrial and cytosolic fractions, respectively. B, mitochondria isolated from the TbTim62-TAP and TbTim54-TAP cells induced for 48 h and from the parental T. brucei (Tb427) cells were lysed with digitonin (1%), and solubilized proteins were used for immunoprecipitation using IgG-Sepharose beads. Proteins from the input (In) 10%, flow-through (ft) 10%, and precipitated (ppt) 50% fractions were analyzed by immunoblot probed with the PAP reagent TAO and Tim17 antibodies. C, mitochondria isolated from the Tim17-TAP cells induced for 48 h and from the Tb427 cells were lysed with digitonin (1%). The soluble proteins were immunoprecipitated with IgG-Sepharose beads. Proteins from the input (In) 20% and precipitated (ppt) 80% were analyzed by immunoblot using the PAP reagent and the Tim17, Hsp70, ATPase β, and TAO antibodies. Molecular masses of the marker proteins are indicated by the side of the blots.