FIGURE 4.
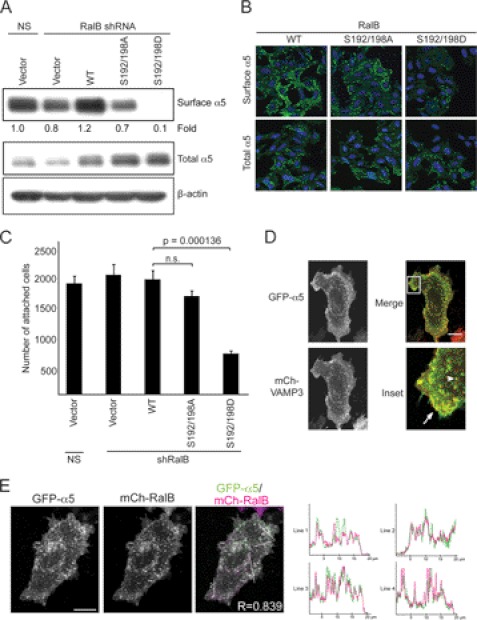
Phosphorylation of RalB regulates the trafficking of α5-integrin. A, RalB phosphorylation alters α5 surface expression. Surface proteins were labeled with biotin in SW480 cells prepared as in Fig. 2A. Biotinylated proteins were isolated on neutravidin resin and resolved by SDS-PAGE. Western blot analyses were performed with anti-α5 antibody to determine the amount of biotinylated, surface-exposed α5. Total cell lysate was blotted with anti-α5 and with anti-β-actin to verify equivalent total protein loading. B, RalB phosphorylation regulates the surface expression of endogenous α5. α5 expression was determined in endogenous RalB-depleted SW480 cells as in Fig. 2A. Representative images are shown of cells that were either nonpermeabilized (for surface α5) or permeabilized (for total α5) and were stained with an Alexa 488-conjugated α5 antibody. C, cellular attachment to fibronectin is reduced in cells expressing phosphomimetic RalB. SW480 cells from Fig. 2A were allowed to attach to fibronectin. Nonadherent cells were removed, and attached cells were quantitated. Values shown are means ± S.E., and an unpaired t test was used to determine significance (p < 0.05). n.s., not significant. D, VAMP3 and α5 co-localize on endosomes and at the plasma membrane. SW480 cells were transiently transfected with mCh-VAMP3 and GFP-α5. Live cells were imaged, and co-localization of VAMP3 with α5 at the plasma membrane (arrow) and on endosomes (arrowhead) was seen. E, RalB and α5 co-localize on endosomes and at the plasma membrane. SW480 cells were transiently transfected with mCh-RalB and GFP-α5. Cells were seeded on glass coverslips for live cell imaging 48 h after transfection. Line scans of the merged image indicate co-localization of RalB with α5 on both internal vesicles and at the plasma membrane. Ten different images were quantitated and a Pearson's correlation coefficient of 0.83 ± 0.001 (S.E.) was calculated. Data are representative of at least two independent experiments. Scale bar, 20 μm.