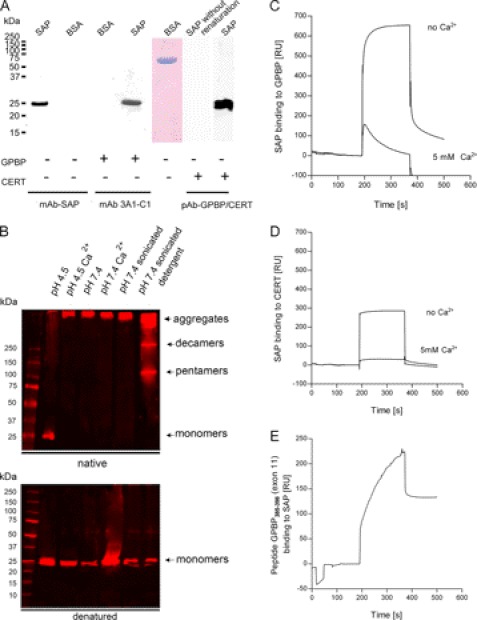
FIGURE 4.
Solid phase interaction of SAP with GPBP. A, far Western blot experiments. Human SAP and BSA were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and renatured. As a negative control for the renaturation, SAP was kept in 6 m urea (SAP without renaturation). After blocking, membranes were incubated with either GPBP or with CERT as a probe using immobilized SAP as bait. Bound proteins were detected with mAb anti-SAP, anti-GPBP (3A1-C1), or a polyclonal antibody against GPBP/CERT. The presence of BSA was confirmed by Coomassie staining. Both GPBP and CERT bound specifically to renatured SAP but not to BSA or denatured SAP. B, SAP aggregation is influenced by pH, the composition of the buffer, and the presence or absence of Ca2+. The treatment of SAP before the Western blot was performed as follows. SAP was diluted at 10 ng/μl in 10 mm sodium acetate buffer, pH 4.5 (lane 1); in sodium acetate buffer, pH 4.5, in the presence of 5 mm calcium (lane 2); in 25 mm HEPES buffer, pH 7.4 (lane 3); in HEPES buffer, pH 7.4, in the presence of 5 mm calcium (lane 4); in HEPES buffer, pH 7.4, followed by sonication with a probe sonicator for three pulses of 30 s each with a 30-s rest on ice between each pulse (lane 5); or in 25 mm HEPES buffer, pH 7.4, followed by sonication as aforementioned in the presence of 0.01% Tween 20 (lane 6). Different percentages of Tween 20 (1, 0.1, and 0.001%) were tested before choosing the optimal at 0.01%. SAP was separated using native PAGE and SDS-PAGE 4–20% gradient gels. The proteins were transferred to nitrocellulose membranes and incubated with anti-SAP antibody. 10 ng/μl SAP diluted in sodium, 10 mm acetate buffer, pH 4.5, and run in native conditions separated unique SAP species of 25 kDa (monomers). 10 ng/μl SAP diluted in HEPES buffer, pH 7.4, sonicated in the presence of 0.01% Tween, and run in native PAGE separated SAP species corresponding to high molecular aggregates of >250 kDa, 250 kDa (decamers), and 100 kDa (pentamers). As expected, independently of the buffer in which SAP has been diluted, in SDS-PAGE, SAP separated as a band of 25 kDa (monomers). C, binding of SAP to immobilized GPBP (215 RU; flow rate, 15 μl/min; injected volume, 60 μl). The binding was recorded in 25 mm HEPES buffer, pH 7.4, 150 mm NaCl. The presence of 5 mm Ca2+ decreased the binding of SAP to immobilized GPBP. D, binding of SAP to immobilized CERT (215 RU; flow rate, 15 μl/min; injected volume, 60 μl). The binding was first recorded in the absence of added Ca2+ in 25 mm HEPES buffer, pH 7.4, 150 mm NaCl. The presence of 5 mm Ca2+ decreased the binding of SAP to immobilized CERT. The general shape of the curves revealed that SAP had very fast association rates with both proteins, although SAP remained bound to GPBP for a longer period at the end of the injection. E, binding of a peptide containing amino acids 385–398 from GPBP exon 11 to immobilized SAP (215 RU; flow rate, 15 μl/min; injected volume, 60 μl) in 25 mm HEPES buffer, pH 7.4, 150 mm NaCl.