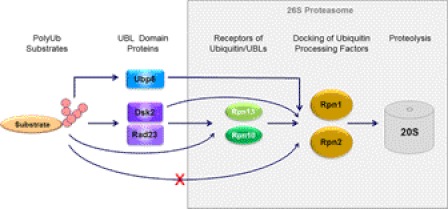
FIGURE 6.
Schematic description of substrate relay. Polyubiquitin-binding proteins such as Dsk2 or Rad23 are transiently associated with proteasomes and thus may serve as shuttles or delivery proteins for polyubiquitin conjugates. These proteins contain an N-terminal UBL (ubiquitin-like) domain through which they dock at the proteasome. The primary UBL receptors for Rad23 and Dsk2 was found to be proteasome subunits Rpn1 (Tables 1–3), although these two UDPs (UBL-domain containing proteins) were also capable of associating directly with two neighboring subunits, Rpn10 and Rpn13 (supplemental Table ST1). Ddi1 apparently also associates with Rpn1, although in this study we were unable to quantify the intensity of their mutual association. Polyubiquitin chains could also bind to Rpn10 and Rpn13 directly (supplemental Table ST1), thereby bypassing the need for shuttles. The relative affinities of these receptors for the different (poly)ubiquitin or UBL signals influence efficiencies of substrate targeting and coordinate their entry into the proteasome. Another UDP, the deubiquitinating enzyme Ubp6, bound only to Rpn1 yet much tighter than transiently associated shuttle proteins, thus behaving more akin to proteasome subunits Rpn10 and Rpn13. The proximal localization of Ubp6 to these polyubiquitin-chain-receptors may also coordinate trimming, processing, or shaving of conjugated chains with substrate preparation and the proteolytic mechanism (see additional details in supplemental Fig. S1).